Species-specific behavioral patterns correlate with differences in synaptic connections between homologous mechanosensory neurons
- PMID: 20135128
- PMCID: PMC2825318
- DOI: 10.1007/s00359-010-0503-y
Species-specific behavioral patterns correlate with differences in synaptic connections between homologous mechanosensory neurons
Abstract
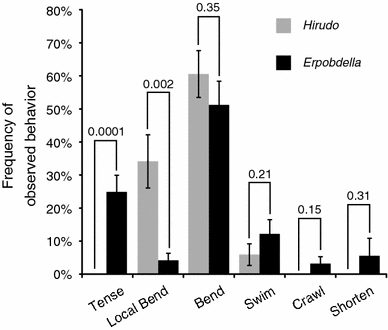
We characterized the behavioral responses of two leech species, Hirudo verbana and Erpobdella obscura, to mechanical skin stimulation and examined the interactions between the pressure mechanosensory neurons (P cells) that innervate the skin. To quantify behavioral responses, we stimulated both intact leeches and isolated body wall preparations from the two species. In response to mechanical stimulation, Hirudo showed local bending behavior, in which the body wall shortened only on the side of the stimulation. Erpobdella, in contrast, contracted both sides of the body in response to touch. To investigate the neuronal basis for this behavioral difference, we studied the interactions between P cells. Each midbody ganglion has four P cells; each cell innervates a different quadrant of the body wall. Consistent with local bending, activating any one P cell in Hirudo elicited polysynaptic inhibitory potentials in the other P cells. In contrast, the P cells in Erpobdella had excitatory polysynaptic connections, consistent with the segment-wide contraction observed in this species. In addition, activating individual P cells caused asymmetrical body wall contractions in Hirudo and symmetrical body wall contractions in Erpobdella. These results suggest that the different behavioral responses in Erpobdella and Hirudo are partly mediated by interactions among mechanosensory cells.
Figures

Similar articles
-
Differing synaptic strengths between homologous mechanosensory neurons.Invert Neurosci. 2014 Sep;14(2):103-11. doi: 10.1007/s10158-014-0168-2. Epub 2014 Feb 14. Invert Neurosci. 2014. PMID: 24526450
-
Coding and adaptation during mechanical stimulation in the leech nervous system.J Physiol. 2000 Dec 15;529 Pt 3(Pt 3):747-62. doi: 10.1111/j.1469-7793.2000.00747.x. J Physiol. 2000. PMID: 11118503 Free PMC article.
-
Convergence of mechanosensory inputs onto neuromodulatory serotonergic neurons in the leech.J Neurobiol. 2003 Mar;54(4):604-17. doi: 10.1002/neu.10184. J Neurobiol. 2003. PMID: 12555272
-
Frequency coding of positional information by an identified neuron, the AP cell, in the leech, Whitmania pigra.Brain Res Bull. 2001 Dec;56(6):511-5. doi: 10.1016/s0361-9230(01)00609-8. Brain Res Bull. 2001. PMID: 11786234
-
Neural control of heartbeat in the leech and in some other invertebrates.Physiol Rev. 1979 Jan;59(1):101-36. doi: 10.1152/physrev.1979.59.1.101. Physiol Rev. 1979. PMID: 220645 Review.
Cited by
-
Multiplexed Population Coding of Stimulus Properties by Leech Mechanosensory Cells.J Neurosci. 2016 Mar 30;36(13):3636-47. doi: 10.1523/JNEUROSCI.1753-15.2016. J Neurosci. 2016. PMID: 27030751 Free PMC article.
-
Transcriptional profiling of identified neurons in leech.BMC Genomics. 2021 Mar 25;22(1):215. doi: 10.1186/s12864-021-07526-0. BMC Genomics. 2021. PMID: 33765928 Free PMC article.
-
Molluscan neurons in culture: shedding light on synapse formation and plasticity.J Mol Histol. 2012 Aug;43(4):383-99. doi: 10.1007/s10735-012-9398-y. Epub 2012 Apr 27. J Mol Histol. 2012. PMID: 22538479 Review.
-
Differing synaptic strengths between homologous mechanosensory neurons.Invert Neurosci. 2014 Sep;14(2):103-11. doi: 10.1007/s10158-014-0168-2. Epub 2014 Feb 14. Invert Neurosci. 2014. PMID: 24526450
-
Premotor Neuron Divergence Reflects Vocal Evolution.J Neurosci. 2018 Jun 6;38(23):5325-5337. doi: 10.1523/JNEUROSCI.0089-18.2018. Epub 2018 May 21. J Neurosci. 2018. PMID: 29875228 Free PMC article.

