Low-temperature FTIR study of multiple K intermediates in the photocycles of bacteriorhodopsin and xanthorhodopsin
- PMID: 20136108
- PMCID: PMC3820168
- DOI: 10.1021/jp908698f
Low-temperature FTIR study of multiple K intermediates in the photocycles of bacteriorhodopsin and xanthorhodopsin
Abstract
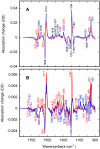
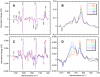
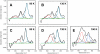
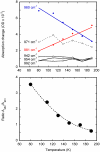
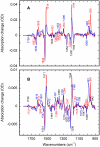
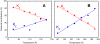
Low-temperature FTIR spectroscopy of bacteriorhodopsin and xanthorhodopsin was used to elucidate the number of K-like bathochromic states, their sequence, and their contributions to the photoequilibrium mixtures created by illumination at 80-180 K. We conclude that in bacteriorhodopsin the photocycle includes three distinct K-like states in the sequence bR (hv)--> I* --> J --> K(0) --> K(E) --> L --> ..., and similarly in xanthorhodopsin. K(0) is the main fraction in the mixture at 77 K that is formed from J. K(0) becomes thermally unstable above approximately 50 K in both proteins. At 77 K, both J-to-K(0) and K(0)-to-K(E) transitions occur and, contrarily to long-standing belief, cryogenic trapping at 77 K does not produce a pure K state but a mixture of the two states, K(0) and K(E), with contributions from K(E) of approximately 15 and approximately 10% in the two retinal proteins, respectively. Raising the temperature leads to increasing conversion of K(0) to K(E), and the two states coexist (without contamination from non-K-like states) in the 80-140 K range in bacteriorhodopsin, and in the 80-190 K range in xanthorhodopsin. Temperature perturbation experiments in these regions of coexistence revealed that, in spite of the observation of apparently stable mixtures of K(0) and K(E), the two states are not in thermally controlled equilibrium. The K(0)-to-K(E) transition is unidirectional, and the partial transformation to K(E) is due to distributed kinetics, which governs the photocycle dynamics at temperatures below approximately 245 K (Dioumaev and Lanyi, Biochemistry 2008, 47, 11125-11133). From spectral deconvolution, we conclude that the K(E) state, which is increasingly present at higher temperatures, is the same intermediate that is detected by time-resolved FTIR prior to its decay, on a time scale of hundreds of nanoseconds at ambient temperature (Dioumaev and Braiman, J. Phys. Chem. B 1997, 101, 1655-1662), into the K(L) state. We were unable to trap the latter separately from K(E) at low temperature, due to the slow distributed kinetics and the increasingly faster overlapping formation of the L state. Formation of the two consecutive K-like states in both proteins is accompanied by distortion of two different weakly bound water molecules: one in K(0), the other in K(E). The first, well-documented in bacteriorhodopsin at 77 K where K(0) dominates, was assigned to water 401 in bacteriorhodopsin. The other water molecule, whose participation has not been described previously, is disturbed on the next step of the photocycle, in K(E), in both proteins. In bacteriorhodopsin, the most likely candidate is water 407. However, unlike bacteriorhodopsin, the crystal structure of xanthorhodopsin lacks homologous weakly bound water molecules.
Figures


Similar articles
-
Switch from conventional to distributed kinetics in the bacteriorhodopsin photocycle.Biochemistry. 2008 Oct 21;47(42):11125-33. doi: 10.1021/bi801247e. Epub 2008 Sep 27. Biochemistry. 2008. PMID: 18821776 Free PMC article.
-
Water structural changes in the L and M photocycle intermediates of bacteriorhodopsin as revealed by time-resolved step-scan Fourier transform infrared (FTIR) spectroscopy.Biochemistry. 2007 Mar 13;46(10):2787-96. doi: 10.1021/bi0616596. Epub 2007 Feb 15. Biochemistry. 2007. PMID: 17300175 Free PMC article.
-
Active internal waters in the bacteriorhodopsin photocycle. A comparative study of the L and M intermediates at room and cryogenic temperatures by infrared spectroscopy.Biochemistry. 2008 Apr 1;47(13):4071-81. doi: 10.1021/bi7024063. Epub 2008 Mar 6. Biochemistry. 2008. PMID: 18321068
-
Trapping and spectroscopic identification of the photointermediates of bacteriorhodopsin at low temperatures.Photochem Photobiol. 2001 May;73(5):453-62. doi: 10.1562/0031-8655(2001)073<0453:tasiot>2.0.co;2. Photochem Photobiol. 2001. PMID: 11367564 Review.
-
Water as a cofactor in the unidirectional light-driven proton transfer steps in bacteriorhodopsin.Photochem Photobiol. 2006 Nov-Dec;82(6):1398-405. doi: 10.1562/2006-01-16-IR-779. Photochem Photobiol. 2006. PMID: 16634652 Review.
Cited by
-
Rational Design of Far-Red Archaerhodopsin-3-Based Fluorescent Genetically Encoded Voltage Indicators: from Elucidation of the Fluorescence Mechanism in Archers to Novel Red-Shifted Variants.ACS Phys Chem Au. 2024 Apr 29;4(4):347-362. doi: 10.1021/acsphyschemau.3c00073. eCollection 2024 Jul 24. ACS Phys Chem Au. 2024. PMID: 39069984 Free PMC article.
-
Tuning the primary reaction of channelrhodopsin-2 by imidazole, pH, and site-specific mutations.Biophys J. 2012 Jun 6;102(11):2649-57. doi: 10.1016/j.bpj.2012.04.034. Epub 2012 Jun 5. Biophys J. 2012. PMID: 22713581 Free PMC article.
-
Photocycle of Exiguobacterium sibiricum rhodopsin characterized by low-temperature trapping in the IR and time-resolved studies in the visible.J Phys Chem B. 2013 Jun 20;117(24):7235-53. doi: 10.1021/jp402430w. Epub 2013 Jun 10. J Phys Chem B. 2013. PMID: 23718558 Free PMC article.
-
Detailed analysis of distorted retinal and its interaction with surrounding residues in the K intermediate of bacteriorhodopsin.Commun Biol. 2023 Feb 17;6(1):190. doi: 10.1038/s42003-023-04554-2. Commun Biol. 2023. PMID: 36808185 Free PMC article.
References
-
- Béjà O, Spudich EN, Spudich JL, Leclerc M, DeLong EF. Nature. 2001;411:786–789. - PubMed
-
- Wang WW, Sineshchekov OA, Spudich EN, Spudich JL. J.Biol.Chem. 2003;278:33985–33991. - PubMed
-
- Sabehi G, Massana R, Bielawski JP, Rosenberg M, DeLong EF, Beja O. Environ.Microbiol. 2003;5:842–849. - PubMed
-
- Brown LS. Photochem.Photobiol.Sci. 2004;3:555–565. - PubMed
-
- Lanyi JK. Annu.Rev.Physiol. 2004;66:665–688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

