Activation of Escherichia coli UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine deacetylase by Fe2+ yields a more efficient enzyme with altered ligand affinity
- PMID: 20136146
- PMCID: PMC2884013
- DOI: 10.1021/bi902066t
Activation of Escherichia coli UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine deacetylase by Fe2+ yields a more efficient enzyme with altered ligand affinity
Abstract
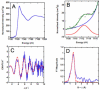
The metal-dependent deacetylase UDP-3-O-[(R)-3-hydroxymyristoyl]-N-acetylglucosamine deacetylase (LpxC) catalyzes the first committed step in lipid A biosynthesis, the hydrolysis of UDP-3-O-myristoyl-N-acetylglucosamine to form UDP-3-O-myristoylglucosamine and acetate. Consequently, LpxC is a target for the development of antibiotics, nearly all of which coordinate the active site metal ion. Here we examine the ability of Fe(2+) to serve as a cofactor for wild-type Escherichia coli LpxC and a mutant enzyme (EcC63A), in which one of the ligands for the inhibitory metal binding site has been removed. LpxC exhibits higher activity (6-8-fold) with a single bound Fe(2+) as the cofactor compared to Zn(2+)-LpxC; both metalloenzymes have a bell-shaped dependence on pH with similar pK(a) values, indicating that at least two ionizations are important for maximal activity. X-ray absorption spectroscopy experiments suggest that the catalytic metal ion bound to Fe(2+)-EcLpxC is five-coordinate, suggesting that catalytic activity may correlate with coordination number. Furthermore, the ligand affinity of Fe(2+)-LpxC compared to the Zn(2+) enzyme is altered by up to 6-fold. In contrast to Zn(2+)-LpxC, the activity of Fe(2+)-LpxC is redox-sensitive, and a time-dependent decrease in activity is observed under aerobic conditions. The LpxC activity of crude E. coli cell lysates is also aerobically sensitive, consistent with the presence of Fe(2+)-LpxC. These data indicate that EcLpxC can use either Fe(2+) or Zn(2+) to activate catalysis in vitro and possibly in vivo, which may allow LpxC to function in E. coli grown under different environmental conditions.
Figures






Similar articles
-
Active site metal ion in UDP-3-O-((R)-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) switches between Fe(II) and Zn(II) depending on cellular conditions.J Biol Chem. 2010 Oct 29;285(44):33788-96. doi: 10.1074/jbc.M110.147173. Epub 2010 Aug 13. J Biol Chem. 2010. PMID: 20709752 Free PMC article.
-
Molecular recognition by Escherichia coli UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase is modulated by bound metal ions.Biochemistry. 2006 Dec 12;45(49):14573-81. doi: 10.1021/bi061625y. Biochemistry. 2006. PMID: 17144651
-
UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase of Escherichia coli is a zinc metalloenzyme.Biochemistry. 1999 Feb 9;38(6):1902-11. doi: 10.1021/bi982339s. Biochemistry. 1999. PMID: 10026271
-
UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase (LpxC) inhibitors: a new class of antibacterial agents.Curr Med Chem. 2012;19(13):2038-50. doi: 10.2174/092986712800167374. Curr Med Chem. 2012. PMID: 22414079 Review.
-
Recent Process in the Inhibitors of UDP-3-O-(R-3-hydroxyacyl)-Nacetylglucosamine Deacetylase (LpxC) Against Gram-Negative Bacteria.Mini Rev Med Chem. 2018;18(4):310-323. doi: 10.2174/1389557516666161013120253. Mini Rev Med Chem. 2018. PMID: 27739357 Review.
Cited by
-
Kinetics and thermodynamics of metal-binding to histone deacetylase 8.Protein Sci. 2015 Mar;24(3):354-65. doi: 10.1002/pro.2623. Epub 2015 Jan 13. Protein Sci. 2015. PMID: 25516458 Free PMC article.
-
The activity and cofactor preferences of N-acetyl-1-D-myo-inosityl-2-amino-2-deoxy-alpha-D-glucopyranoside deacetylase (MshB) change depending on environmental conditions.J Biol Chem. 2011 Jun 10;286(23):20275-82. doi: 10.1074/jbc.M111.234229. Epub 2011 Apr 20. J Biol Chem. 2011. PMID: 21507949 Free PMC article.
-
Control of lipopolysaccharide biosynthesis by FtsH-mediated proteolysis of LpxC is conserved in enterobacteria but not in all gram-negative bacteria.J Bacteriol. 2011 Mar;193(5):1090-7. doi: 10.1128/JB.01043-10. Epub 2010 Dec 30. J Bacteriol. 2011. PMID: 21193611 Free PMC article.
-
Recent advances in small molecule LpxC inhibitors against gram-negative bacteria (2014-2024).Front Microbiol. 2025 Feb 10;16:1541379. doi: 10.3389/fmicb.2025.1541379. eCollection 2025. Front Microbiol. 2025. PMID: 40041875 Free PMC article. Review.
-
Structures of metal-substituted human histone deacetylase 8 provide mechanistic inferences on biological function.Biochemistry. 2010 Jun 22;49(24):5048-56. doi: 10.1021/bi1005046. Biochemistry. 2010. PMID: 20545365 Free PMC article.
References
-
- Wyckoff TJO, Raetz CRH, Jackman JE. Antibacterial and anti-inflammatory agents that target endotoxin. Trends in Microbiology. 1998;6:154–159. - PubMed
-
- White RJ, Margolis PS, Trias J, Yuan ZY. Targeting metalloenzymes: a strategy that works. Current Opinion in Pharmacology. 2003;3:502–507. - PubMed
-
- Young K, Silver LL, Bramhill D, Cameron P, Eveland SS, Raetz CRH, Hyland SA, Anderson MS. The envA permeability cell division gene of Escherichia coli encodes the second enzyme of lipid A biosynthesis - UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine deacetylase. Journal of Biological Chemistry. 1995;270:30384–30391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

