Characterization of ex vivo-generated bovine and human cartilage by immunohistochemical, biochemical, and magnetic resonance imaging analyses
- PMID: 20136403
- PMCID: PMC2947949
- DOI: 10.1089/ten.TEA.2009.0717
Characterization of ex vivo-generated bovine and human cartilage by immunohistochemical, biochemical, and magnetic resonance imaging analyses
Abstract
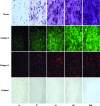
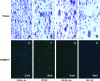
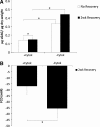
Osteoarthritis (OA) is a prevalent age-associated disease involving altered chondrocyte homeostasis and cartilage degeneration. The avascular nature of cartilage and the altered chondrocyte phenotype characteristic of OA severely limit the capacity for in vivo tissue regeneration. Cell- and tissue-based repair has the potential to revolutionize treatment of OA, but those approaches have exhibited limited clinical success to date. In this study, we test the hypothesis that bovine and human chondrocytes in a collagen type I scaffold will form hyaline cartilage ex vivo with immunohistochemical, biochemical, and magnetic resonance (MR) endpoints similar to the original native cartilage. Chondrocytes were isolated from 1- to 3-week-old calf knee cartilage or from cartilage obtained from human total knee arthroplasties, suspended in 2.7 mg/mL collagen I, and plated as 300 microL spot cultures with 5 x 10(6) each. Medium formulations were varied, including the amount of serum, the presence or absence of ascorbate, and treatments with cytokines. Bovine chondrocytes generated metachromatic territorial and interstitial matrix and accumulated type II collagen over time. Type VI collagen was confined primarily to the pericellular region. The ex vivo-formed bovine cartilage contained more chondroitin sulfate per dry weight than native cartilage. Human chondrocytes remained viable and generated metachromatic territorial matrix, but were unable to support interstitial matrix accumulation. MR analysis of ex vivo-formed bovine cartilage revealed evidence of progressively maturing matrix, but MR-derived indices of tissue quality did not reach those of native cartilage. We conclude that the collagen-spot culture model supports formation and maturation of three-dimensional hyaline cartilage from active bovine chondrocytes. Future studies will focus on determining the capacity of human chondrocytes to show comparable tissue formation.
Figures






References
-
- Chen C.T. Fishbein K.W. Torzilli P.A. Hilger A. Spencer R.G. Horton W.E., Jr. Matrix fixed-charge density as determined by magnetic resonance microscopy of bioreactor-derived hyaline cartilage correlates with biochemical and biomechanical properties. Arthritis Rheum. 2003;48:1047. - PubMed
-
- Shulz R.M. Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36:539. - PubMed
-
- Almqvist K.F. Dhollander A.A. Verdonk P.C. Forsyth R. Verdonk R. Verbruggen G. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am J Sports Med. 2009;37:1920. - PubMed
-
- Chajra H. Rousseau C.F. Cortial D. Ronzière M.C. Herbage D. Mallein-Gerin F. Freyria A.M. Collagen-based biomaterials and cartilage engineering. Application to osteochondral defects. Biomed Mater Eng. 2008;18:S33. - PubMed
-
- Mohan N. Nair P. A synthetic scaffold favouring chondrogenic phenotype over a natural scaffold. Tissue Eng Part A. 2010;16:373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

