Oxidative protein folding and the Quiescin-sulfhydryl oxidase family of flavoproteins
- PMID: 20136510
- PMCID: PMC2959182
- DOI: 10.1089/ars.2010.3098
Oxidative protein folding and the Quiescin-sulfhydryl oxidase family of flavoproteins
Abstract
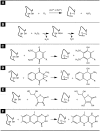
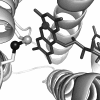
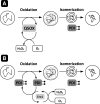
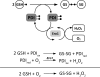
Flavin-linked sulfhydryl oxidases participate in the net generation of disulfide bonds during oxidative protein folding in the endoplasmic reticulum. Members of the Quiescin-sulfhydryl oxidase (QSOX) family catalyze the facile direct introduction of disulfide bonds into unfolded reduced proteins with the reduction of molecular oxygen to generate hydrogen peroxide. Current progress in dissecting the mechanism of QSOX enzymes is reviewed, with emphasis on the CxxC motifs in the thioredoxin and Erv/ALR domains and the involvement of the flavin prosthetic group. The tissue distribution and intra- and extracellular location of QSOX enzymes are discussed, and suggestions for the physiological role of these enzymes are presented. The review compares the substrate specificity and catalytic efficiency of the QSOX enzymes with members of the Ero1 family of flavin-dependent sulfhydryl oxidases: enzymes believed to play key roles in disulfide generation in yeast and higher eukaryotes. Finally, limitations of our current understanding of disulfide generation in metazoans are identified and questions posed for the future.
Figures




Similar articles
-
Generating disulfides with the Quiescin-sulfhydryl oxidases.Biochim Biophys Acta. 2008 Apr;1783(4):567-77. doi: 10.1016/j.bbamcr.2007.10.002. Epub 2007 Oct 12. Biochim Biophys Acta. 2008. PMID: 17980160 Free PMC article. Review.
-
Going through the barrier: coupled disulfide exchange reactions promote efficient catalysis in quiescin sulfhydryl oxidase.J Biol Chem. 2014 Feb 21;289(8):5274-84. doi: 10.1074/jbc.M113.536219. Epub 2013 Dec 30. J Biol Chem. 2014. PMID: 24379406 Free PMC article.
-
Quiescin sulfhydryl oxidase from Trypanosoma brucei: catalytic activity and mechanism of a QSOX family member with a single thioredoxin domain.Biochemistry. 2010 Mar 9;49(9):2075-85. doi: 10.1021/bi902222s. Biochemistry. 2010. PMID: 20121244 Free PMC article.
-
Erv2 and quiescin sulfhydryl oxidases: Erv-domain enzymes associated with the secretory pathway.Antioxid Redox Signal. 2012 Apr 15;16(8):800-8. doi: 10.1089/ars.2011.4450. Epub 2012 Jan 11. Antioxid Redox Signal. 2012. PMID: 22142242 Review.
-
Flavin-linked Erv-family sulfhydryl oxidases release superoxide anion during catalytic turnover.Biochemistry. 2012 Jan 10;51(1):265-72. doi: 10.1021/bi201672h. Epub 2011 Dec 16. Biochemistry. 2012. PMID: 22148553 Free PMC article.
Cited by
-
Proteolytic processing of QSOX1A ensures efficient secretion of a potent disulfide catalyst.Biochem J. 2013 Sep 1;454(2):181-90. doi: 10.1042/BJ20130360. Biochem J. 2013. PMID: 23713614 Free PMC article.
-
Multivalency in the inhibition of oxidative protein folding by arsenic(III) species.Biochemistry. 2015 Jan 20;54(2):612-21. doi: 10.1021/bi501360e. Epub 2014 Dec 30. Biochemistry. 2015. PMID: 25506675 Free PMC article.
-
Disulfide bonds in ER protein folding and homeostasis.Curr Opin Cell Biol. 2011 Apr;23(2):167-75. doi: 10.1016/j.ceb.2010.10.012. Epub 2010 Dec 7. Curr Opin Cell Biol. 2011. PMID: 21144725 Free PMC article. Review.
-
Vitamin K epoxide reductase contributes to protein disulfide formation and redox homeostasis within the endoplasmic reticulum.Mol Biol Cell. 2012 Jun;23(11):2017-27. doi: 10.1091/mbc.E12-02-0102. Epub 2012 Apr 11. Mol Biol Cell. 2012. PMID: 22496424 Free PMC article.
-
Quiescin/sulfhydryl oxidase 1b (QSOX1b) induces migration and proliferation of vascular smooth muscle cells by distinct redox pathways.Arch Biochem Biophys. 2020 Jan 15;679:108220. doi: 10.1016/j.abb.2019.108220. Epub 2019 Dec 5. Arch Biochem Biophys. 2020. PMID: 31812669 Free PMC article.
References
-
- Appenzeller–Herzog C. Ellgaard L. The human PDI family: Versatility packed into a single fold. Biochim Biophys Acta. 2008;1783:535–548. - PubMed
-
- Bach RD. Dmitrenko O. Thorpe C. Mechanism of thiolate-disulfide interchange reactions in biochemistry. J Org Chem. 2008;73:12–21. - PubMed
-
- Banhegyi G. Csala M. Szarka A. Varsanyi M. Benedetti A. Mandl J. Role of ascorbate in oxidative protein folding. Biofactors. 2003;17:37–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

