Neonatal programming by neuroimmune challenge: effects on responses and tolerance to septic doses of lipopolysaccharide in adult male and female rats
- PMID: 20136690
- PMCID: PMC3522740
- DOI: 10.1111/j.1365-2826.2010.01967.x
Neonatal programming by neuroimmune challenge: effects on responses and tolerance to septic doses of lipopolysaccharide in adult male and female rats
Abstract
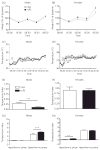
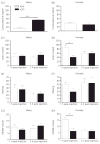
A mild immune challenge experienced during the neonatal period leads to attenuated febrile responses to a similar challenge experienced later in life. However, the immune response to an endotoxin differs depending upon the severity of the challenge and it is not clear whether a neonatal immune challenge will also affect responses to a severe, potentially life-threatening stimulus, such as sepsis. In the present study, we examined the effects of a neonatal immune challenge with lipopolysaccharide (LPS) on adult sickness responses, as well as the development of endotoxin tolerance, to a septic dose (1 or 3 mg/kg) of the same LPS in male and female rats. We demonstrate significant differences, particularly in males, in the fever profiles of neonatally LPS-treated rats compared to neonatally saline-treated controls. Specifically, male rats treated neonatally with LPS have reduced hypothermic and enhanced hyperthermic responses to both septic doses of LPS in adulthood. A somewhat different profile is seen in females, with neonatally LPS-treated females having reduced hypothermia and enhanced hyperthermia compared to controls with 1 mg/kg but no differences with 3 mg/kg LPS. The results obtained demonstrate that alterations in innate immune responses previously reported for low doses of LPS can, for the most part, also be observed after severe immune challenge in later life.
Figures



Similar articles
-
Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics.J Physiol. 2006 Mar 15;571(Pt 3):695-701. doi: 10.1113/jphysiol.2005.102939. Epub 2006 Jan 19. J Physiol. 2006. PMID: 16423854 Free PMC article.
-
Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults.FASEB J. 2005 Sep;19(11):1519-21. doi: 10.1096/fj.04-3569fje. Epub 2005 Jun 22. FASEB J. 2005. PMID: 15972802
-
Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge.Physiol Behav. 2006 Jan 30;87(1):126-34. doi: 10.1016/j.physbeh.2005.09.008. Epub 2005 Nov 21. Physiol Behav. 2006. PMID: 16300807
-
Neonatal programming of innate immune function.Am J Physiol Endocrinol Metab. 2011 Jan;300(1):E11-8. doi: 10.1152/ajpendo.00516.2010. Epub 2010 Nov 2. Am J Physiol Endocrinol Metab. 2011. PMID: 21045175 Free PMC article. Review.
-
Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis.J Endotoxin Res. 2003;9(1):3-24. doi: 10.1179/096805103125001298. J Endotoxin Res. 2003. PMID: 12691614 Review.
Cited by
-
Nutritionally mediated programming of the developing immune system.Adv Nutr. 2011 Sep;2(5):377-95. doi: 10.3945/an.111.000570. Epub 2011 Sep 6. Adv Nutr. 2011. PMID: 22332080 Free PMC article. Review.
-
Sex effects on neurodevelopmental outcomes of innate immune activation during prenatal and neonatal life.Horm Behav. 2012 Aug;62(3):228-36. doi: 10.1016/j.yhbeh.2012.03.015. Epub 2012 Apr 6. Horm Behav. 2012. PMID: 22516179 Free PMC article. Review.
-
Enduring influence of pubertal stressors on behavioral response to hormones in female mice.Horm Behav. 2013 Jul;64(2):390-8. doi: 10.1016/j.yhbeh.2013.01.015. Horm Behav. 2013. PMID: 23998680 Free PMC article. Review.
-
Lifetime Modulation of the Pain System via Neuroimmune and Neuroendocrine Interactions.Front Immunol. 2017 Mar 13;8:276. doi: 10.3389/fimmu.2017.00276. eCollection 2017. Front Immunol. 2017. PMID: 28348566 Free PMC article. Review.
-
Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats.Biol Sex Differ. 2012 Jun 14;3(1):15. doi: 10.1186/2042-6410-3-15. Biol Sex Differ. 2012. PMID: 22697211 Free PMC article.
References
-
- Jiang Q, Detolla L, Singh IS, Gatdula L, Fitzgerald B, van Rooijen N, Cross AS, Hasday JD. Exposure to febrile temperature upregulates expression of pyrogenic cytokines in endotoxin-challenged mice. Am J Physiol. 1999;276:R1653–R1660. - PubMed
-
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. - PubMed
-
- Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann NY Acad Sci. 1998;856:224–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

