High frequencies of less differentiated and more proliferative WT1-specific CD8+ T cells in bone marrow in tumor-bearing patients: an important role of bone marrow as a secondary lymphoid organ
- PMID: 20136847
- PMCID: PMC11158461
- DOI: 10.1111/j.1349-7006.2009.01468.x
High frequencies of less differentiated and more proliferative WT1-specific CD8+ T cells in bone marrow in tumor-bearing patients: an important role of bone marrow as a secondary lymphoid organ
Abstract
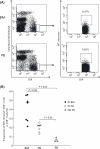
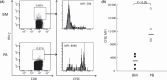
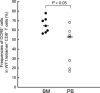
In tumor-bearing patients, tumor-associated antigen (TAA)-specific CTLs are spontaneously induced as a result of immune response to TAAs and play an important role in anti-tumor immunity. Wilms' tumor gene 1 (WT1) is overexpressed in various types of tumor and WT1 protein is a promising pan-TAA because of its high immunogenicity. In this study, to clarify the immune response to the WT1 antigen, WT1-specific CD8(+) T cells that were spontaneously induced in patients with solid tumor were comparatively analyzed in both bone marrow (BM) and peripheral blood (PB). WT1-specific CD8(+) T cells more frequently existed in BM than in PB, whereas frequencies of naïve (CCR7(+) CD45RA(+)), central memory (CCR7(+) CD45RA-), effector-memory (CCR7- CD45RA(-)), and effector (CCR7- CD45RA(+)) subsets were not significantly different between BM and PB. However, analysis of these subsets for the expression of CD57 and CD28, which were associated with differentiation, revealed that effector-memory and effector subsets of the WT1-specific CD8(+) T cells in BM had less differentiated phenotypes and more proliferative potential than those in PB. Furthermore, CD107a/b functional assay for WT1 peptide-specific cytotoxic potential and carboxyfluorescein diacetate succinimidyl ester dilution assay for WT1 peptide-specific proliferation also showed that WT1-specific CD8(+) T cells in BM were less cytotoxic and more proliferative in response to WT1 peptide than those in PB. These results implied that BM played an important role as a secondary lymphoid organ in tumor-bearing patients. Preferential residence of WT1-specific CD8(+) T cells in BM could be, at least in part, explained by higher expression of chemokine receptor CCR5, whose ligand was expressed on BM fibroblasts on the WT1-specific CD8(+) T cells in BM, compared to those in PB. These results should provide us with an insight into WT1-specific immune response in tumor-bearing patients and give us an idea of enhancement of clinical response in WT1 protein-targeted immunotherapy.
Figures



Similar articles
-
Differentiation of human CD8(+) T cells from a memory to memory/effector phenotype.J Immunol. 2002 Jun 1;168(11):5538-50. doi: 10.4049/jimmunol.168.11.5538. J Immunol. 2002. PMID: 12023349
-
Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells.J Immunol. 2002 Mar 1;168(5):2225-32. doi: 10.4049/jimmunol.168.5.2225. J Immunol. 2002. PMID: 11859109
-
WT1-specific CD8 + cytotoxic T cells with the capacity for antigen-specific expansion accumulate in the bone marrow in MDS.Int J Hematol. 2021 May;113(5):723-734. doi: 10.1007/s12185-021-03083-0. Epub 2021 Jan 27. Int J Hematol. 2021. PMID: 33502734
-
Wilms tumor gene WT1 peptide-based immunotherapy induced a minimal response in a patient with advanced therapy-resistant multiple myeloma.Int J Hematol. 2007 Dec;86(5):414-7. doi: 10.1007/BF02983998. Int J Hematol. 2007. PMID: 18192109
-
Biased usage of BV gene families of T-cell receptors of WT1 (Wilms' tumor gene)-specific CD8+ T cells in patients with myeloid malignancies.Cancer Sci. 2010 Mar;101(3):594-600. doi: 10.1111/j.1349-7006.2009.01453.x. Epub 2009 Dec 4. Cancer Sci. 2010. PMID: 20132220 Free PMC article.
Cited by
-
Mapping of novel peptides of WT-1 and presenting HLA alleles that induce epitope-specific HLA-restricted T cells with cytotoxic activity against WT-1(+) leukemias.Blood. 2012 Aug 23;120(8):1633-46. doi: 10.1182/blood-2011-11-394619. Epub 2012 May 23. Blood. 2012. PMID: 22623625 Free PMC article.
-
Long-term persistence of limited HTLV-I Tax-specific cytotoxic T cell clones in a patient with adult T cell leukemia/lymphoma after allogeneic stem cell transplantation.J Clin Immunol. 2012 Dec;32(6):1340-52. doi: 10.1007/s10875-012-9729-5. Epub 2012 Jul 5. J Clin Immunol. 2012. PMID: 22763862
-
Counteracting Immunosuppression in the Tumor Microenvironment by Oncolytic Newcastle Disease Virus and Cellular Immunotherapy.Int J Mol Sci. 2022 Oct 27;23(21):13050. doi: 10.3390/ijms232113050. Int J Mol Sci. 2022. PMID: 36361831 Free PMC article. Review.
-
Bone marrow vaccination: a novel approach to enhance antigen specific antitumor immunity.Vaccine. 2011 Nov 3;29(47):8599-605. doi: 10.1016/j.vaccine.2011.09.022. Epub 2011 Sep 25. Vaccine. 2011. PMID: 21951877 Free PMC article.
-
Editorial: T cell memory, bone marrow, and aging: the good news.J Leukoc Biol. 2012 Feb;91(2):185-7. doi: 10.1189/jlb.0811438. J Leukoc Biol. 2012. PMID: 22293941 Free PMC article.
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3: 991–8. - PubMed
-
- Malmberg KJ, Ljunggren HG. Escape from immune‐ and nonimmune‐mediated tumor surveillance. Semin Cancer Biol 2006; 16: 16–31. - PubMed
-
- Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin‐like receptor G1 on antigen‐specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol 2005; 174: 6088–94. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

