Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis
- PMID: 20137099
- PMCID: PMC2827393
- DOI: 10.1186/1465-9921-11-16
Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis
Abstract
Background: No effective treatment for acute lung injury and fibrosis currently exists. Aim of this study was to investigate the time-dependent effect of bone marrow-derived mesenchymal stem cells (BMDMSCs) on bleomycin (BLM)-induced acute lung injury and fibrosis and nitric oxide metabolites and inflammatory cytokine production.
Methods: BMDMSCs were transferred 4 days after BLM inhalation. Wet/dry ratio, bronchoalveolar lavage cell profiles, histologic changes and deposition of collagen were analyzed.
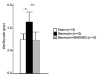
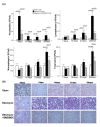
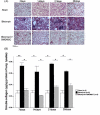
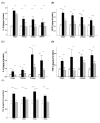
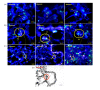
Results: Nitrite, nitrate and cytokines were measured weekly through day 28. At day 7, the wet/dry ratio, neutrophilic inflammation, and amount of collagen were elevated in BLM-treated rats compared to sham rats (p = 0.05-0.002). Levels nitrite, nitrate, IL-1beta, IL-6, TNF-alpha, TGF-beta and VEGF were also higher at day 7 (p < 0.05). Degree of lymphocyte and macrophage infiltration increased steadily over time. BMDMSC transfer significantly reduced the BLM-induced increase in wet/dry ratio, degree of neutrophilic infiltration, collagen deposition, and levels of the cytokines, nitrite, and nitrate to those in sham-treated rats (p < 0.05). Fluorescence in situ hybridization localized the engrafted cells to areas of lung injury.
Conclusion: Systemic transfer of BMDMSCs effectively reduced the BLM-induced lung injury and fibrosis through the down-regulation of nitric oxide metabolites, and proinflammatory and angiogenic cytokines.
Figures


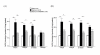
Similar articles
-
[Effects of andrographolide on the concentration of cytokines in BALF and the expressions of type I and III collagen mRNA in lung tissue in bleomycin-induced rat pulmonary fibrosis].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011 Jul;27(7):725-9. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2011. PMID: 21722520 Chinese.
-
Nicorandil ameliorates bleomycin-induced pulmonary fibrosis in rats through modulating eNOS, iNOS, TXNIP and HIF-1α levels.Life Sci. 2020 Apr 1;246:117423. doi: 10.1016/j.lfs.2020.117423. Epub 2020 Feb 11. Life Sci. 2020. PMID: 32057902
-
Exogenous nitric oxide enhances the prophylactic effect of aminoguanidine, a preferred iNOS inhibitor, on bleomycin-induced fibrosis in the lung: Implications for the direct roles of the NO molecule in vivo.Nitric Oxide. 2017 Nov 1;70:31-41. doi: 10.1016/j.niox.2017.07.005. Epub 2017 Jul 27. Nitric Oxide. 2017. PMID: 28757441
-
Inhibition of bleomycin-induced pulmonary fibrosis by bone marrow-derived mesenchymal stem cells might be mediated by decreasing MMP9, TIMP-1, INF-γ and TGF-β.Cell Biochem Funct. 2015 Aug;33(6):356-66. doi: 10.1002/cbf.3118. Epub 2015 Jul 15. Cell Biochem Funct. 2015. PMID: 26178702
-
Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways.Biomed Pharmacother. 2017 May;89:1297-1309. doi: 10.1016/j.biopha.2017.03.005. Epub 2017 Mar 17. Biomed Pharmacother. 2017. PMID: 28320097
Cited by
-
Current therapeutic strategies for respiratory diseases using mesenchymal stem cells.MedComm (2020). 2021 Sep 2;2(3):351-380. doi: 10.1002/mco2.74. eCollection 2021 Sep. MedComm (2020). 2021. PMID: 34766151 Free PMC article. Review.
-
Mesenchymal Stem Cell Therapy of Pulmonary Fibrosis: Improvement with Target Combination.Cell Transplant. 2018 Nov;27(11):1581-1587. doi: 10.1177/0963689718787501. Epub 2018 Jul 11. Cell Transplant. 2018. PMID: 29991279 Free PMC article.
-
Optical imaging of subacute airway remodeling and adipose stem cell engraftment after airway injury.Biomed Opt Express. 2013 Dec 20;5(1):312-21. doi: 10.1364/BOE.5.000312. eCollection 2013 Dec 20. Biomed Opt Express. 2013. PMID: 24466496 Free PMC article.
-
Therapeutic effects of amniotic fluid-derived mesenchymal stromal cells on lung injury in rats with emphysema.Respir Res. 2014 Oct 16;15(1):120. doi: 10.1186/s12931-014-0120-3. Respir Res. 2014. PMID: 25319435 Free PMC article.
-
Bone Marrow Mononuclear Cell Transplantation Restores Inflammatory Balance of Cytokines after ST Segment Elevation Myocardial Infarction.PLoS One. 2015 Dec 21;10(12):e0145094. doi: 10.1371/journal.pone.0145094. eCollection 2015. PLoS One. 2015. PMID: 26690350 Free PMC article. Clinical Trial.
References
-
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;4186893:41–49. doi: 10.1038/nature00870. - DOI - PubMed
-
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

