The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion
- PMID: 20137952
- PMCID: PMC3163294
- DOI: 10.1016/j.cub.2009.12.035
The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion
Abstract
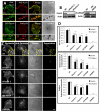
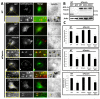
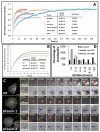
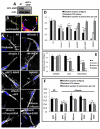
Fascin is an actin-bundling protein involved in filopodia assembly and cancer invasion and metastasis of multiple epithelial cancer types. Fascin forms stable actin bundles with slow dissociation kinetics in vitro and is regulated by phosphorylation of serine 39 by protein kinase C (PKC). Cancer cells use invasive finger-like protrusions termed invadopodia to invade into and degrade extracellular matrix. Invadopodia have highly dynamic actin that is assembled by both Arp2/3 complex and formins; they also contain components of membrane trafficking machinery such as dynamin and cortactin and have been compared with focal adhesions and podosomes. We show that fascin is an integral component of invadopodia and that it is important for the stability of actin in invadopodia. The phosphorylation state of fascin at S39, a PKC site, contributes to its regulation at invadopodia. We further implicate fascin in invasive migration into collagen I-Matrigel gels and particularly in cell types that use an elongated mesenchymal type of motility in 3D. We provide a potential molecular mechanism for how fascin increases the invasiveness of cancer cells, and we compare invadopodia with invasive filopod-like structures in 3D.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J Mol Biol. 2001;310:351–366. - PubMed
-
- Adams JC. Roles of fascin in cell adhesion and motility. Curr Opin Cell Biol. 2004;16:590–596. - PubMed
-
- Baldassarre M, Ayala I, Beznoussenko G, Giacchetti G, Machesky LM, Luini A, Buccione R. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–1231. - PubMed
-
- Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-related formins are required for invadopodia formation and invasion of breast tumor cells. Cancer Res. 2009;69:2792–2800. - PubMed
-
- Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

