Acetylcholinesterase: from 3D structure to function
- PMID: 20138030
- PMCID: PMC2894301
- DOI: 10.1016/j.cbi.2010.01.042
Acetylcholinesterase: from 3D structure to function
Abstract
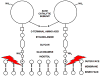
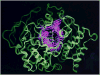
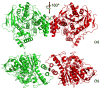
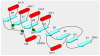
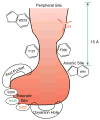
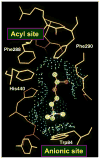
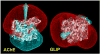
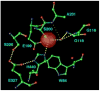
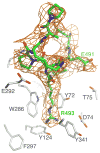
By rapid hydrolysis of the neurotransmitter, acetylcholine, acetylcholinesterase terminates neurotransmission at cholinergic synapses. Acetylcholinesterase is a very fast enzyme, functioning at a rate approaching that of a diffusion-controlled reaction. The powerful toxicity of organophosphate poisons is attributed primarily to their potent inhibition of acetylcholinesterase. Acetylcholinesterase inhibitors are utilized in the treatment of various neurological disorders, and are the principal drugs approved thus far by the FDA for management of Alzheimer's disease. Many organophosphates and carbamates serve as potent insecticides, by selectively inhibiting insect acetylcholinesterase. The determination of the crystal structure of Torpedo californica acetylcholinesterase permitted visualization, for the first time, at atomic resolution, of a binding pocket for acetylcholine. It also allowed identification of the active site of acetylcholinesterase, which, unexpectedly, is located at the bottom of a deep gorge lined largely by aromatic residues. The crystal structure of recombinant human acetylcholinesterase in its apo-state is similar in its overall features to that of the Torpedo enzyme; however, the unique crystal packing reveals a novel peptide sequence which blocks access to the active-site gorge.
Copyright (c) 2010 Elsevier Ireland Ltd. All rights reserved.
Figures








Similar articles
-
Targeted oxidation of Torpedo californica acetylcholinesterase by singlet oxygen: identification of N-formylkynurenine tryptophan derivatives within the active-site gorge of its complex with the photosensitizer methylene blue.Biochem J. 2012 Nov 15;448(1):83-91. doi: 10.1042/BJ20120992. Biochem J. 2012. PMID: 22888904 Free PMC article.
-
Crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site.J Am Chem Soc. 2008 Jun 25;130(25):7856-61. doi: 10.1021/ja7109822. Epub 2008 May 31. J Am Chem Soc. 2008. PMID: 18512913 Free PMC article.
-
Active-site gorge and buried water molecules in crystal structures of acetylcholinesterase from Torpedo californica.J Mol Biol. 2000 Feb 18;296(2):713-35. doi: 10.1006/jmbi.1999.3468. J Mol Biol. 2000. PMID: 10669619
-
Acetylcholinesterase: how is structure related to function?Chem Biol Interact. 2008 Sep 25;175(1-3):3-10. doi: 10.1016/j.cbi.2008.05.035. Epub 2008 Jun 6. Chem Biol Interact. 2008. PMID: 18586019 Review.
-
A preliminary comparison of structural models for catalytic intermediates of acetylcholinesterase.Chem Biol Interact. 1999 May 14;119-120:43-52. doi: 10.1016/s0009-2797(99)00012-5. Chem Biol Interact. 1999. PMID: 10421437 Review.
Cited by
-
Development of Rapid and High-Precision Colorimetric Device for Organophosphorus Pesticide Detection Based on Microfluidic Mixer Chip.Micromachines (Basel). 2021 Mar 9;12(3):290. doi: 10.3390/mi12030290. Micromachines (Basel). 2021. PMID: 33803445 Free PMC article.
-
Merged Tacrine-Based, Multitarget-Directed Acetylcholinesterase Inhibitors 2015-Present: Synthesis and Biological Activity.Int J Mol Sci. 2020 Aug 19;21(17):5965. doi: 10.3390/ijms21175965. Int J Mol Sci. 2020. PMID: 32825138 Free PMC article. Review.
-
Novel tacrine-benzofuran hybrids as potential multi-target drug candidates for the treatment of Alzheimer's Disease.J Enzyme Inhib Med Chem. 2020 Dec;35(1):211-226. doi: 10.1080/14756366.2019.1689237. J Enzyme Inhib Med Chem. 2020. PMID: 31760822 Free PMC article.
-
Identification of new allosteric sites and modulators of AChE through computational and experimental tools.J Enzyme Inhib Med Chem. 2018 Dec;33(1):1034-1047. doi: 10.1080/14756366.2018.1476502. J Enzyme Inhib Med Chem. 2018. PMID: 29873262 Free PMC article.
-
Fine Tuning of Cholinesterase and Glutathione-S-Transferase Activities by Organoruthenium(II) Complexes.Biomedicines. 2021 Sep 16;9(9):1243. doi: 10.3390/biomedicines9091243. Biomedicines. 2021. PMID: 34572429 Free PMC article.
References
-
- Barnard EA. Neuromuscular transmission - enzymatic destruction of acetylcholine. In: Hubbard JI, editor. The Peripheral Nervous System. Plenum; New York: 1974. pp. 201–224.
-
- Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev. 1987;87:955–975.
-
- Taylor P. Anticholinesterase agents. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. The Pharmacological Basis of Therapeutics. 9. McGraw-Hill; New York: 1996. pp. 161–176.
-
- Greenblatt HM, Dvir H, Silman I, Sussman JL. Acetylcholinesterase: a multifaceted target for structure-based drug design of anticholinesterase agents for the treatment of Alzheimer’s disease. J Mol Neurosci. 2003;20:369–384. - PubMed
-
- Dougherty DA, Stauffer DA. Acetylcholine binding by a synthetic receptor: implications for biological recognition. Science. 1990;250:1558–1560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

