Nanotopographical modification: a regulator of cellular function through focal adhesions
- PMID: 20138244
- PMCID: PMC2965469
- DOI: 10.1016/j.nano.2010.01.009
Nanotopographical modification: a regulator of cellular function through focal adhesions
Abstract
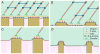
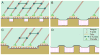
As materials technology and the field of biomedical engineering advances, the role of cellular mechanisms, in particular adhesive interactions with implantable devices, becomes more relevant in both research and clinical practice. A key tenet of medical device design has evolved from the exquisite ability of biological systems to respond to topographical features or chemical stimuli, a process that has led to the development of next-generation biomaterials for a wide variety of clinical disorders. In vitro studies have identified nanoscale features as potent modulators of cellular behavior through the onset of focal adhesion formation. The focus of this review is on the recent developments concerning the role of nanoscale structures on integrin-mediated adhesion and cellular function with an emphasis on the generation of medical constructs with regenerative applications.
From the clinical editor: In this review, recent developments related to the role of nanoscale structures on integrin-mediated adhesion and cellular function is discussed, with an emphasis on regenerative applications.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
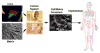



Similar articles
-
Focal adhesions in osteoneogenesis.Proc Inst Mech Eng H. 2010 Dec;224(12):1441-53. doi: 10.1243/09544119JEIM775. Proc Inst Mech Eng H. 2010. PMID: 21287830 Free PMC article.
-
The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography.Biomaterials. 2007 Apr;28(10):1787-97. doi: 10.1016/j.biomaterials.2006.12.020. Epub 2006 Dec 21. Biomaterials. 2007. PMID: 17218005
-
Mechanisms of hepatocyte attachment to keratin biomaterials.Biomaterials. 2011 Oct;32(30):7555-61. doi: 10.1016/j.biomaterials.2011.06.061. Epub 2011 Jul 23. Biomaterials. 2011. PMID: 21782237
-
Cell sensing of physical properties at the nanoscale: Mechanisms and control of cell adhesion and phenotype.Acta Biomater. 2016 Jan;30:26-48. doi: 10.1016/j.actbio.2015.11.027. Epub 2015 Nov 17. Acta Biomater. 2016. PMID: 26596568 Review.
-
Focal adhesions: structure and dynamics.Biol Cell. 2000 Oct;92(7):477-94. doi: 10.1016/s0248-4900(00)01101-1. Biol Cell. 2000. PMID: 11229600 Review.
Cited by
-
Nanostructured surfaces of dental implants.Int J Mol Sci. 2013 Jan 17;14(1):1918-31. doi: 10.3390/ijms14011918. Int J Mol Sci. 2013. PMID: 23344062 Free PMC article. Review.
-
Mechano-bactericidal actions of nanostructured surfaces.Nat Rev Microbiol. 2021 Jan;19(1):8-22. doi: 10.1038/s41579-020-0414-z. Epub 2020 Aug 17. Nat Rev Microbiol. 2021. PMID: 32807981 Review.
-
Mesh-like electrospun membrane loaded with atorvastatin facilitates cutaneous wound healing by promoting the paracrine function of mesenchymal stem cells.Stem Cell Res Ther. 2022 May 7;13(1):190. doi: 10.1186/s13287-022-02865-5. Stem Cell Res Ther. 2022. PMID: 35526075 Free PMC article.
-
Neuronal contact guidance and YAP signaling on ultra-small nanogratings.Sci Rep. 2020 Feb 28;10(1):3742. doi: 10.1038/s41598-020-60745-5. Sci Rep. 2020. PMID: 32111918 Free PMC article.
-
Osteoconductive potential of barrier nanoSiO2 PLGA membranes functionalized by plasma enhanced chemical vapour deposition.Biomed Res Int. 2014;2014:253590. doi: 10.1155/2014/253590. Epub 2014 May 4. Biomed Res Int. 2014. PMID: 24883304 Free PMC article.
References
-
- Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. - PubMed
-
- Curtis A. Tutorial on the biology of nanotopography. IEEE Trans Nanobiosci. 2004;3:293–5. - PubMed
-
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour. I. Simple step cues. Development. 1987;99:439–48. - PubMed
-
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Cell guidance by ultrafine topography in vitro. J Cell Sci. 1991;99(Pt 1):73–7. - PubMed
-
- Tanaka M, Takayama A, Ito E, Sunami H, Yamamoto S, Shimomura M. Effect of pore size of self-organized honeycomb-patterned polymer films on spreading, focal adhesion, proliferation, and function of endothelial cells. J Nanosci Nanotechnol. 2007;7:763–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

