ABC transporters, atherosclerosis and inflammation
- PMID: 20138281
- PMCID: PMC2888932
- DOI: 10.1016/j.atherosclerosis.2010.01.011
ABC transporters, atherosclerosis and inflammation
Abstract
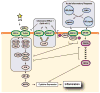
Atherosclerosis, driven by inflamed lipid-laden lesions, can occlude the coronary arteries and lead to myocardial infarction. This chronic disease is a major and expensive health burden. However, the body is able to mobilize and excrete cholesterol and other lipids, thus preventing atherosclerosis by a process termed reverse cholesterol transport (RCT). Insight into the mechanism of RCT has been gained by the study of two rare syndromes caused by the mutation of ABC transporter loci. In Tangier disease, loss of ABCA1 prevents cells from exporting cholesterol and phospholipid, thus resulting in the build-up of cholesterol in the peripheral tissues and a loss of circulating HDL. Consistent with HDL being an athero-protective particle, Tangier patients are more prone to develop atherosclerosis. Likewise, sitosterolemia is another inherited syndrome associated with premature atherosclerosis. Here mutations in either the ABCG5 or G8 loci, prevents hepatocytes and enterocytes from excreting cholesterol and plant sterols, including sitosterol, into the bile and intestinal lumen. Thus, ABCG5 and G8, which from a heterodimer, constitute a transporter that excretes cholesterol and dietary sterols back into the gut, while ABCA1 functions to export excess cell cholesterol and phospholipid during the biogenesis of HDL. Interestingly, a third protein, ABCG1, that has been shown to have anti-atherosclerotic activity in mice, may also act to transfer cholesterol to mature HDL particles. Here we review the relationship between the lipid transport activities of these proteins and their anti-atherosclerotic effect, particularly how they may reduce inflammatory signaling pathways. Of particular interest are recent reports that indicate both ABCA1 and ABCG1 modulate cell surface cholesterol levels and inhibit its partitioning into lipid rafts. Given lipid rafts may provide platforms for innate immune receptors to respond to inflammatory signals, it follows that loss of ABCA1 and ABCG1 by increasing raft content will increase signaling through these receptors, as has been experimentally demonstrated. Moreover, additional reports indicate ABCA1, and possibly SR-BI, another HDL receptor, may directly act as anti-inflammatory receptors independent of their lipid transport activities. Finally, we give an update on the progress and pitfalls of therapeutic approaches that seek to stimulate the flux of lipids through the RCT pathway.
Copyright 2010 Elsevier Ireland Ltd. All rights reserved.
Figures

References
-
- Fitzgerald ML, Xavier R, Haley KJ, et al. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48(3):621–32. - PubMed
-
- Wymann MP, Schneiter R. Lipid signalling in disease. Nat Rev Mol Cell Biol. 2008;9(2):162–76. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

