Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations
- PMID: 20138358
- PMCID: PMC2847433
- DOI: 10.1016/j.biomaterials.2010.01.035
Inducing local T cell apoptosis with anti-Fas-functionalized polymeric coatings fabricated via surface-initiated photopolymerizations
Abstract
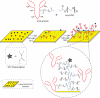
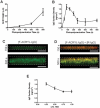
Cell encapsulation has long been investigated as a means to achieve transplant immunoprotection as it creates a physical barrier between allograft tissue and host immune cells. Encapsulation with passive barrier materials alone, however, is generally insufficient to protect donor tissue from rejection, because small cytotoxic molecules produced by activated T cells can diffuse readily into the capsule and mediate allograft death. As a means to provide bioactive protection for polymeric encapsulation devices, we investigated a functionalized polymeric coating that mimics a natural T cell regulation pathway. T cells are regulated in vivo via Fas, a well-known 'death receptor,' whereby effector cells express Fas ligand and elicit T cell apoptosis upon binding the Fas receptor on a T cell surface. Anti-Fas antibodies are capable of replicating this effect and induce T cell apoptosis in solution. Here, an iniferter-based living radical polymerization was utilized to fabricate surface-anchored polymer chains containing poly(ethylene glycol) with covalently incorporated pendant anti-Fas antibody. Using this reaction mechanism, we demonstrate fabrication conditions that yield surface densities in excess of 1.5 ng/cm(2) of incorporated therapeutic, as detected by ELISA. Additionally, we show that coatings containing anti-Fas antibody induced significant T cell apoptosis, 21+/-2% of cells, after 24h. Finally, the incorporation of a T cell adhesion ligand, intracellular adhesion molecule-1, along with anti-Fas antibody, yielded even higher levels of apoptosis, 34+/-1% of T cells, compared to either signal alone.
Published by Elsevier Ltd.
Figures


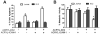
Similar articles
-
Functionalized PEG hydrogels through reactive dip-coating for the formation of immunoactive barriers.Biomaterials. 2011 Sep;32(26):6204-12. doi: 10.1016/j.biomaterials.2011.04.049. Epub 2011 Jun 11. Biomaterials. 2011. PMID: 21658759 Free PMC article.
-
The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand.J Exp Med. 1996 Sep 1;184(3):1075-82. doi: 10.1084/jem.184.3.1075. J Exp Med. 1996. PMID: 9064324 Free PMC article.
-
Fas aggregation does not correlate with Fas-mediated apoptosis.J Immunol. 2001 Jul 1;167(1):82-9. doi: 10.4049/jimmunol.167.1.82. J Immunol. 2001. PMID: 11418635
-
On the role and significance of Fas (Apo-1/CD95) ligand (FasL) expression in immune privileged tissues and cancer cells using multiple myeloma as a model.Leuk Lymphoma. 1998 Nov;31(5-6):477-90. doi: 10.3109/10428199809057607. Leuk Lymphoma. 1998. PMID: 9922038 Review.
-
Soluble HLA class I molecules/CD8 ligation trigger apoptosis of CD8+ cells by Fas/Fas-ligand interaction.ScientificWorldJournal. 2002 Feb 12;2:421-3. doi: 10.1100/tsw.2002.122. ScientificWorldJournal. 2002. PMID: 12806026 Free PMC article. Review.
Cited by
-
Controlled release strategies for modulating immune responses to promote tissue regeneration.J Control Release. 2015 Dec 10;219:155-166. doi: 10.1016/j.jconrel.2015.08.014. Epub 2015 Aug 8. J Control Release. 2015. PMID: 26264833 Free PMC article. Review.
-
Extracellular Matrix-Based Biomaterials for Cardiovascular Tissue Engineering.J Cardiovasc Dev Dis. 2021 Oct 22;8(11):137. doi: 10.3390/jcdd8110137. J Cardiovasc Dev Dis. 2021. PMID: 34821690 Free PMC article. Review.
-
Immunomodulating Hydrogels as Stealth Platform for Drug Delivery Applications.Pharmaceutics. 2022 Oct 21;14(10):2244. doi: 10.3390/pharmaceutics14102244. Pharmaceutics. 2022. PMID: 36297679 Free PMC article. Review.
-
Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3D Bone Tissue.Adv Healthc Mater. 2017 Aug;6(16):10.1002/adhm.201700015. doi: 10.1002/adhm.201700015. Epub 2017 May 19. Adv Healthc Mater. 2017. PMID: 28524375 Free PMC article.
-
Combinatorial drug delivery approaches for immunomodulation.Adv Drug Deliv Rev. 2017 May 15;114:161-174. doi: 10.1016/j.addr.2017.05.013. Epub 2017 May 19. Adv Drug Deliv Rev. 2017. PMID: 28532690 Free PMC article. Review.
References
-
- Wollert KC, Drexler H. Cell-based therapy for heart failure. Curr Opin Cardiol. 2006;21(3):234–239. - PubMed
-
- Emerich DF, Winn SR. Immunoisolation cell therapy for CNS diseases. Crit Rev Ther Drug Carrier Syst. 2001;18(3):265–298. - PubMed
-
- Lee MK, Bae YH. Cell transplantation for endocrine disorders. Adv Drug Deliv Rev. 2000;42(12):103–120. - PubMed
-
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210(4472):908–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

