Characterization of topographical effects on macrophage behavior in a foreign body response model
- PMID: 20138663
- PMCID: PMC2837101
- DOI: 10.1016/j.biomaterials.2010.01.074
Characterization of topographical effects on macrophage behavior in a foreign body response model
Abstract
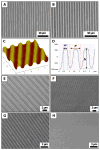
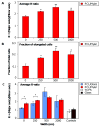
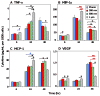
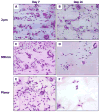
Current strategies to limit macrophage adhesion, fusion and fibrous capsule formation in the foreign body response have focused on modulating material surface properties. We hypothesize that topography close to biological scale, in the micron and nanometric range, provides a passive approach without bioactive agents to modulate macrophage behavior. In our study, topography-induced changes in macrophage behavior was examined using parallel gratings (250 nm-2 mum line width) imprinted on poly(epsilon-caprolactone) (PCL), poly(lactic acid) (PLA) and poly(dimethyl siloxane) (PDMS). RAW 264.7 cell adhesion and elongation occurred maximally on 500 nm gratings compared to planar controls over 48 h. TNF-alpha and VEGF secretion levels by RAW 264.7 cells showed greatest sensitivity to topographical effects, with reduced levels observed on larger grating sizes at 48 h. In vivo studies at 21 days showed reduced macrophage adhesion density and degree of high cell fusion on 2 mum gratings compared to planar controls. It was concluded that topography affects macrophage behavior in the foreign body response on all polymer surfaces examined. Topography-induced changes, independent of surface chemistry, did not reveal distinctive patterns but do affect cell morphology and cytokine secretion in vitro, and cell adhesion in vivo particularly on larger size topography compared to planar controls.
Copyright 2010 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures





Similar articles
-
Theoretical analysis of in vivo macrophage adhesion and foreign body giant cell formation on polydimethylsiloxane, low density polyethylene, and polyetherurethanes.J Biomed Mater Res. 1994 Jan;28(1):73-9. doi: 10.1002/jbm.820280110. J Biomed Mater Res. 1994. PMID: 8126032
-
In vivobiocompatibility and biodegradability of poly(lactic acid)/poly(ε-caprolactone) blend compatibilized with poly(ε-caprolactone-b-tetrahydrofuran) in Wistar rats.Biomed Phys Eng Express. 2021 Mar 15;7(3). doi: 10.1088/2057-1976/abeb5a. Biomed Phys Eng Express. 2021. PMID: 33652429
-
Lymphocytes and the foreign body response: lymphocyte enhancement of macrophage adhesion and fusion.J Biomed Mater Res A. 2005 Aug 1;74(2):222-9. doi: 10.1002/jbm.a.30313. J Biomed Mater Res A. 2005. PMID: 15948198
-
Foreign body reaction to biomaterials.Semin Immunol. 2008 Apr;20(2):86-100. doi: 10.1016/j.smim.2007.11.004. Epub 2007 Dec 26. Semin Immunol. 2008. PMID: 18162407 Free PMC article. Review.
-
Beyond Encapsulation: Exploring Macrophage-Fibroblast Cross Talk in Implant-Induced Fibrosis.Tissue Eng Part B Rev. 2024 Dec;30(6):596-606. doi: 10.1089/ten.TEB.2023.0300. Epub 2024 Mar 27. Tissue Eng Part B Rev. 2024. PMID: 38420650 Review.
Cited by
-
In vivo Implantation of a Bovine-Derived Collagen Membrane Leads to Changes in the Physiological Cellular Pattern of Wound Healing by the Induction of Multinucleated Giant Cells: An Adverse Reaction?Front Bioeng Biotechnol. 2018 Aug 14;6:104. doi: 10.3389/fbioe.2018.00104. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 30155464 Free PMC article.
-
Effect of Porous Substrate Topographies on Cell Dynamics: A Computational Study.ACS Biomater Sci Eng. 2023 Oct 9;9(10):5666-5678. doi: 10.1021/acsbiomaterials.3c01008. Epub 2023 Sep 15. ACS Biomater Sci Eng. 2023. PMID: 37713253 Free PMC article.
-
Effects of TiO2 nanotube layers on RAW 264.7 macrophage behaviour and bone morphogenetic protein-2 expression.Cell Prolif. 2013 Dec;46(6):685-94. doi: 10.1111/cpr.12072. Cell Prolif. 2013. PMID: 24460720 Free PMC article.
-
In vivo micro-computed tomography evaluation of radiopaque, polymeric device degradation in normal and inflammatory environments.Acta Biomater. 2024 Jun;181:222-234. doi: 10.1016/j.actbio.2024.04.031. Epub 2024 Apr 20. Acta Biomater. 2024. PMID: 38648912 Free PMC article.
-
The directional migration and differentiation of mesenchymal stem cells toward vascular endothelial cells stimulated by biphasic calcium phosphate ceramic.Regen Biomater. 2018 Jun;5(3):129-139. doi: 10.1093/rb/rbx028. Epub 2017 Oct 31. Regen Biomater. 2018. PMID: 29977596 Free PMC article.
References
-
- Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29:2941–53. - PubMed
-
- Ratner BD, Bryant SJ. Biomaterials: where we have been and where we are going. Annu Rev Biomed Eng. 2004;6:41–75. - PubMed
-
- Atienza C, Maloney WJ. Highly cross-linked polyethylene bearing surfaces in total hip arthroplasty. J Surg Orthop Adv. 2008;17:27–33. - PubMed
-
- Santerre JP, Woodhouse K, Laroche G, Labow RS. Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials. 2005;26:7457–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

