Bioactive hydrogels made from step-growth derived PEG-peptide macromers
- PMID: 20138664
- PMCID: PMC2837100
- DOI: 10.1016/j.biomaterials.2010.01.058
Bioactive hydrogels made from step-growth derived PEG-peptide macromers
Abstract
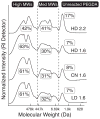
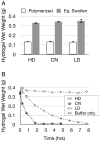
Synthetic hydrogels based on poly(ethylene glycol) (PEG) have been used as biomaterials for cell biology and tissue engineering investigations. Bioactive PEG-based gels have largely relied on heterobifunctional or multi-arm PEG precursors that can be difficult to synthesize and characterize or expensive to obtain. Here, we report an alternative strategy, which instead uses inexpensive and readily available PEG precursors to simplify reactant sourcing. This new approach provides a robust system in which to probe cellular interactions with the microenvironment. We used the step-growth polymerization of PEG diacrylate (PEGDA, 3400Da) with bis-cysteine matrix metalloproteinase (MMP)-sensitive peptides via Michael-type addition to form biodegradable photoactive macromers of the form acrylate-PEG-(peptide-PEG)(m)-acrylate. The molecular weight (MW) of these macromers is controlled by the stoichiometry of the reaction, with a high proportion of resultant macromer species greater than 500kDa. In addition, the polydispersity of these materials was nearly identical for three different MMP-sensitive peptide sequences subjected to the same reaction conditions. When photopolymerized into hydrogels, these high MW materials exhibit increased swelling and sensitivity to collagenase-mediated degradation as compared to previously published PEG hydrogel systems. Cell-adhesive acrylate-PEG-CGRGDS was synthesized similarly and its immobilization and stability in solid hydrogels was characterized with a modified Lowry assay. To illustrate the functional utility of this approach in a biological setting, we applied this system to develop materials that promote angiogenesis in an ex vivo aortic arch explant assay. We demonstrate the formation and invasion of new sprouts mediated by endothelial cells into the hydrogels from embedded embryonic chick aortic arches. Furthermore, we show that this capillary sprouting and three-dimensional migration of endothelial cells can be tuned by engineering the MMP-susceptibility of the hydrogels and the presence of functional immobilized adhesive ligands (CGRGDS vs. CGRGES peptide). The facile chemistry described and significant cellular responses observed suggest the usefulness of these materials in a variety of in vitro and ex vivo biologic investigations, and may aid in the design or refinement of material systems for a range of tissue engineering approaches.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
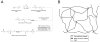


References
-
- Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. - PubMed
-
- Hill-West JL, Chowdhury SM, Sawhney AS, Pathak CP, Dunn RC, Hubbell JA. Prevention of postoperative adhesions in the rat by in situ photopolymerization of bioresorbable hydrogel barriers. Obstet Gynecol. 1994;83:59–64. - PubMed
-
- West J, Hubbell J. Polymeric biomaterials with degradation sites for proteases involved in cell migration. Macromolecules. 1999;32:241–4.
-
- Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

