Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway
- PMID: 20138864
- PMCID: PMC2878914
- DOI: 10.1016/j.yexcr.2010.02.001
Cross-regulation of signaling pathways: an example of nuclear hormone receptors and the canonical Wnt pathway
Abstract
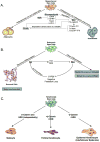
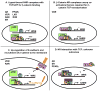
Predicting the potential physiological outcome(s) of any given molecular pathway is complex because of cross-talk with other pathways. This is particularly evident in the case of the nuclear hormone receptor and canonical Wnt pathways, which regulate cell growth and proliferation, differentiation, apoptosis, and metastatic potential in numerous tissues. These pathways are known to intersect at many levels: in the intracellular space, at the membrane, in the cytoplasm, and within the nucleus. The outcomes of these interactions are important in the control of stem cell differentiation and maintenance, feedback loops, and regulating oncogenic potential. The aim of this review is to demonstrate the importance of considering pathway cross-talk when predicting functional outcomes of signaling, using nuclear hormone receptor/canonical Wnt pathway cross-talk as an example.
Figures
Similar articles
-
The androgen receptor can signal through Wnt/beta-Catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens.BMC Cell Biol. 2008 Jan 24;9:4. doi: 10.1186/1471-2121-9-4. BMC Cell Biol. 2008. PMID: 18218096 Free PMC article.
-
Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know?Endocr Rev. 2005 Dec;26(7):898-915. doi: 10.1210/er.2003-0034. Epub 2005 Aug 26. Endocr Rev. 2005. PMID: 16126938 Review.
-
Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer.J Cell Biochem. 2006 Oct 1;99(2):402-10. doi: 10.1002/jcb.20983. J Cell Biochem. 2006. PMID: 16741972 Review.
-
Wnt signaling from membrane to nucleus: β-catenin caught in a loop.Int J Biochem Cell Biol. 2012 Jun;44(6):847-50. doi: 10.1016/j.biocel.2012.03.001. Epub 2012 Mar 14. Int J Biochem Cell Biol. 2012. PMID: 22433990 Review.
-
Roles and regulation of Wnt signaling and beta-catenin in prostate cancer.Cancer Lett. 2006 Jun 8;237(1):22-32. doi: 10.1016/j.canlet.2005.06.004. Epub 2005 Jul 14. Cancer Lett. 2006. PMID: 16023783 Review.
Cited by
-
Circadian disruption accelerates tumor growth and angio/stromagenesis through a Wnt signaling pathway.PLoS One. 2010 Dec 23;5(12):e15330. doi: 10.1371/journal.pone.0015330. PLoS One. 2010. PMID: 21203463 Free PMC article.
-
Constitutive scaffolding of multiple Wnt enhanceosome components by Legless/BCL9.Elife. 2017 Mar 15;6:e20882. doi: 10.7554/eLife.20882. Elife. 2017. PMID: 28296634 Free PMC article.
-
Physiopathological aspects of the Wnt/β-catenin signaling pathway in the male reproductive system.Spermatogenesis. 2013 Jan 1;3(1):e23181. doi: 10.4161/spmg.23181. Spermatogenesis. 2013. PMID: 23687614 Free PMC article.
-
Targeting of oncogenic signaling pathways by berberine for treatment of colorectal cancer.Med Oncol. 2020 Apr 17;37(6):49. doi: 10.1007/s12032-020-01367-9. Med Oncol. 2020. PMID: 32303850 Review.
-
TCF/LEFs and Wnt signaling in the nucleus.Cold Spring Harb Perspect Biol. 2012 Nov 1;4(11):a007906. doi: 10.1101/cshperspect.a007906. Cold Spring Harb Perspect Biol. 2012. PMID: 23024173 Free PMC article. Review.
References
-
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3 beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996;272:1023–1026. - PubMed
-
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. - PubMed
-
- Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683–690. - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of b-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

