The SLO3 sperm-specific potassium channel plays a vital role in male fertility
- PMID: 20138882
- PMCID: PMC2875124
- DOI: 10.1016/j.febslet.2010.02.005
The SLO3 sperm-specific potassium channel plays a vital role in male fertility
Abstract
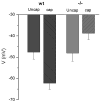
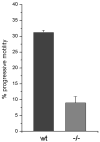
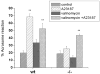
Here we show a unique example of male infertility conferred by a gene knockout of the sperm-specific, pH-dependent SLO3 potassium channel. In striking contrast to wild-type sperm which undergo membrane hyperpolarization during capacitation, we found that SLO3 mutant sperm undergo membrane depolarization. Several defects in SLO3 mutant sperm are evident under capacitating conditions, including impaired motility, a bent "hairpin" shape, and failure to undergo the acrosome reaction (AR). The failure of AR is rescued by valinomycin which hyperpolarizes mutant sperm. Thus SLO3 is the principal potassium channel responsible for capacitation-induced hyperpolarization, and membrane hyperpolarization is crucial to the AR.
Published by Elsevier B.V.
Figures



References
-
- Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–3516. - PubMed
-
- Salkoff L, Butler A, Ferreira G, Santi CM, Wei A. High conductance Potassium Channels of the Slo Family. Nature Reviews Neuroscience. 2006;5:921–931. - PubMed
-
- Vredenburgh-Wilberg WL, Parrish JJ. Intracellular pH of bovine sperm increases during capacitation. Mol Reprod Biol. 1995;40 (4):490–502. - PubMed
-
- Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+, Cl−, and HCO3− dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173:510–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

