Mapping Alzheimer's disease progression in 1309 MRI scans: power estimates for different inter-scan intervals
- PMID: 20139010
- PMCID: PMC2846999
- DOI: 10.1016/j.neuroimage.2010.01.104
Mapping Alzheimer's disease progression in 1309 MRI scans: power estimates for different inter-scan intervals
Abstract
Neuroimaging centers and pharmaceutical companies are working together to evaluate treatments that might slow the progression of Alzheimer's disease (AD), a common but devastating late-life neuropathology. Recently, automated brain mapping methods, such as tensor-based morphometry (TBM) of structural MRI, have outperformed cognitive measures in their precision and power to track disease progression, greatly reducing sample size estimates for drug trials. In the largest TBM study to date, we studied how sample size estimates for tracking structural brain changes depend on the time interval between the scans (6-24 months). We analyzed 1309 brain scans from 91 probable AD patients (age at baseline: 75.4+/-7.5 years) and 189 individuals with mild cognitive impairment (MCI; 74.6+/-7.1 years), scanned at baseline, 6, 12, 18, and 24 months. Statistical maps revealed 3D patterns of brain atrophy at each follow-up scan relative to the baseline; numerical summaries were used to quantify temporal lobe atrophy within a statistically-defined region-of-interest. Power analyses revealed superior sample size estimates over traditional clinical measures. Only 80, 46, and 39 AD patients were required for a hypothetical clinical trial, at 6, 12, and 24 months respectively, to detect a 25% reduction in average change using a two-sided test (alpha=0.05, power=80%). Correspondingly, 106, 79, and 67 subjects were needed for an equivalent MCI trial aiming for earlier intervention. A 24-month trial provides most power, except when patient attrition exceeds 15-16%/year, in which case a 12-month trial is optimal. These statistics may facilitate clinical trial design using voxel-based brain mapping methods such as TBM.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures

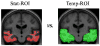

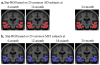
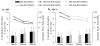


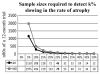
Comment in
-
Bias in tensor based morphometry Stat-ROI measures may result in unrealistic power estimates.Neuroimage. 2011 Jul 1;57(1):1-4. doi: 10.1016/j.neuroimage.2010.11.092. Epub 2011 Feb 22. Neuroimage. 2011. PMID: 21349340 Free PMC article.
References
-
- Apostolova LG, Dutton RA, Dinov ID, Hayashi KM, Toga AW, Cummings JL, Thompson PM. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

