Triptolide reduces cyst formation in a neonatal to adult transition Pkd1 model of ADPKD
- PMID: 20139063
- PMCID: PMC2902895
- DOI: 10.1093/ndt/gfp777
Triptolide reduces cyst formation in a neonatal to adult transition Pkd1 model of ADPKD
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD), a major cause of end-stage renal failure, results from genetic mutation of either polycystin-1 (Pkd1) or polycystin-2 (Pkd2). In order to develop novel therapies to treat the advancement of disease progression, numerous rodent models of different genetic backgrounds are available to study cyst development.
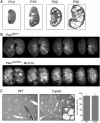
Methods: Here, a Pkd1-floxed inducible mouse model using the interferon responsive Mx1Cre-recombinase was utilized to test the effect of the small molecule triptolide. Relative to other Pkd1 inactivation models, cyst progression in this neonatal to adult transition model is attenuated. Following the characterization of inducible cyst formation in these mice, the development of kidney cysts from triptolide or vehicle-treated animals was analysed.
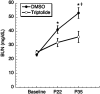
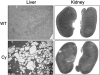
Results: Although Pkd1 deletion on postnatal Days P10 and P12 resulted in numerous cysts by P35, daily injections with triptolide beginning on Day P16 significantly reduced the total number of cysts per kidney, with a pronounced effect on the number of microcysts and the overall cystic burden. Additionally, renal function as assessed by blood urea nitrogen levels was also improved in triptolide-treated mice at both the P22 and P35 time points. As the Pkd1(flox/flox);Mx1Cre model has not been previously used for drug development studies, the feasibility of a 6-month adult Pkd1 inactivation study was also tested. While kidney cyst formation was minimal and focal in nature, livers of these Pkd1-deficient mice were severely cystic, enlarged and pale.
Conclusions: These results suggest that the Pkd1(flox/flox);Mx1Cre model of ADPKD is amenable to short-term kidney cyst formation drug studies; however, it may be problematic for long-term therapeutic research where widespread liver cysts and fibrosis could compromise drug metabolism.
Figures

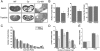
Similar articles
-
Triptolide reduces cystogenesis in a model of ADPKD.J Am Soc Nephrol. 2008 Sep;19(9):1659-62. doi: 10.1681/ASN.2008030259. Epub 2008 Jul 23. J Am Soc Nephrol. 2008. PMID: 18650476 Free PMC article.
-
Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model.Am J Physiol Renal Physiol. 2022 Jan 1;322(1):F27-F41. doi: 10.1152/ajprenal.00298.2021. Epub 2021 Nov 22. Am J Physiol Renal Physiol. 2022. PMID: 34806449
-
Cyclin-Dependent Kinase 1 Activity Is a Driver of Cyst Growth in Polycystic Kidney Disease.J Am Soc Nephrol. 2021 Jan;32(1):41-51. doi: 10.1681/ASN.2020040511. Epub 2020 Oct 12. J Am Soc Nephrol. 2021. PMID: 33046531 Free PMC article.
-
Translational research in ADPKD: lessons from animal models.Nat Rev Nephrol. 2014 Oct;10(10):587-601. doi: 10.1038/nrneph.2014.137. Epub 2014 Aug 19. Nat Rev Nephrol. 2014. PMID: 25137562 Review.
-
Autosomal dominant polycystic kidney disease: recent advances in pathogenesis and potential therapies.Clin Exp Nephrol. 2013 Jun;17(3):317-26. doi: 10.1007/s10157-012-0741-0. Epub 2012 Nov 29. Clin Exp Nephrol. 2013. PMID: 23192769 Review.
Cited by
-
The role of the cilium in normal and abnormal cell cycles: emphasis on renal cystic pathologies.Cell Mol Life Sci. 2013 Jun;70(11):1849-74. doi: 10.1007/s00018-012-1052-z. Epub 2012 Jul 11. Cell Mol Life Sci. 2013. PMID: 22782110 Free PMC article. Review.
-
Triptolide as an Alternative to IVIG Therapy for Kawasaki Disease in a Mouse Model.Balkan Med J. 2013 Jun;30(2):225-8. doi: 10.5152/balkanmedj.2013.7963. Epub 2013 Jun 1. Balkan Med J. 2013. PMID: 25207104 Free PMC article.
-
Pyrrolidine dithiocarbamate reduces the progression of total kidney volume and cyst enlargement in experimental polycystic kidney disease.Physiol Rep. 2014 Dec 11;2(12):e12196. doi: 10.14814/phy2.12196. Print 2014 Dec 1. Physiol Rep. 2014. PMID: 25501440 Free PMC article.
-
Investigating the Catalytic Activity of Glycosyltransferase on Quercetin from Tripterygium wilfordii.ACS Omega. 2020 Jan 13;5(3):1414-1421. doi: 10.1021/acsomega.9b02919. eCollection 2020 Jan 28. ACS Omega. 2020. PMID: 32010813 Free PMC article.
-
Autophagy in renal diseases.Pediatr Nephrol. 2016 May;31(5):737-52. doi: 10.1007/s00467-015-3134-2. Epub 2015 Jul 4. Pediatr Nephrol. 2016. PMID: 26141928 Review.
References
-
- Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. - PubMed
-
- Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. - PubMed
-
- Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, et al. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. - PubMed
-
- Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17:179–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

