Down-regulation of MyoD by calpain 3 promotes generation of reserve cells in C2C12 myoblasts
- PMID: 20139084
- PMCID: PMC2857084
- DOI: 10.1074/jbc.M109.063966
Down-regulation of MyoD by calpain 3 promotes generation of reserve cells in C2C12 myoblasts
Abstract
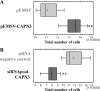
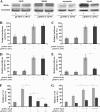
Calpain 3 is a calcium-dependent cysteine protease that is primarily expressed in skeletal muscle and is implicated in limb girdle muscular dystrophy type 2A. To date, its best characterized function is located within the sarcomere, but this protease is found in other cellular compartments, which suggests that it exerts multiple roles. Here, we present evidence that calpain 3 is involved in the myogenic differentiation process. In the course of in vitro culture of myoblasts to fully differentiated myotubes, a population of quiescent undifferentiated "reserve cells" are maintained. These reserve cells are closely related to satellite cells responsible for adult muscle regeneration. In the present work, we observe that reserve cells express higher levels of endogenous Capn3 mRNA than proliferating myoblasts. We show that calpain 3 participates in the establishment of the pool of reserve cells by decreasing the transcriptional activity of the key myogenic regulator MyoD via proteolysis independently of the ubiquitin-proteasome degradation pathway. Our results identify calpain 3 as a potential new player in the muscular regeneration process by promoting renewal of the satellite cell compartment.
Figures


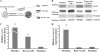




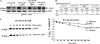
References
-
- Hawke T. J., Garry D. J. (2001) J. Appl. Physiol. 91, 534–551 - PubMed
-
- Cooper R. N., Tajbakhsh S., Mouly V., Cossu G., Buckingham M., Butler-Browne G. S. (1999) J. Cell Sci. 112, 2895–2901 - PubMed
-
- Cornelison D. D., Wold B. J. (1997) Dev. Biol. 191, 270–283 - PubMed
-
- Smith C. K., 2nd, Janney M. J., Allen R. E. (1994) J. Cell. Physiol. 159, 379–385 - PubMed
-
- Baroffio A., Bochaton-Piallat M. L., Gabbiani G., Bader C. R. (1995) Differentiation 59, 259–268 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

