Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation
- PMID: 20139144
- PMCID: PMC2837375
- DOI: 10.1677/JOE-09-0414
Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation
Abstract
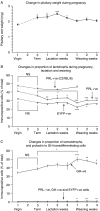
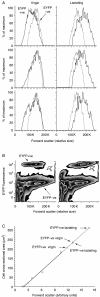
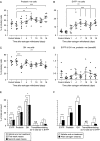
In rats, a shift from somatotroph dominance to lactotroph dominance during pregnancy and lactation is well reported. Somatotroph to lactotroph transdifferentiation and increased lactotroph mitotic activity are believed to account for this and associated pituitary hypertrophy. A combination of cell death and transdifferentiation away from the lactotroph phenotype has been reported to restore non-pregnant pituitary proportions after weaning. To attempt to confirm that a similar process occurs in mice, we generated and used a transgenic reporter mouse model (prolactin (PRL)-Cre/ROSA26-expression of yellow fluorescent protein (EYFP)) in which PRL promoter activity at any time resulted in permanent, stable, and highly specific EYFP. Triple immunochemistry for GH, PRL, and EYFP was used to quantify EYFP+ve, PRL-ve, and GH+ve cell populations during pregnancy and lactation, and for up to 3 weeks after weaning, and concurrent changes in cell size were estimated. At all stages, the EYFP reporter was expressed in 80% of the lactotrophs, but in fewer than 1% of other pituitary cell types, indicating that transdifferentiation from those lactotrophs where reporter expression was activated is extremely rare. Contrary to expectations, no increase in the lactotroph/somatotroph ratio was seen during pregnancy and lactation, whether assessed by immunochemistry for the reporter or PRL: findings confirmed by PRL immunochemistry in non-transgenic mice. Mammosomatotrophs were rarely encountered at the age group studied. Individual EYFP+ve cell volumes increased significantly by mid-lactation compared with virgin animals. This, in combination with a modest and non-cell type-specific estrogen-induced increase in mitotic activity, could account for pregnancy-induced changes in overall pituitary size.
Figures


Similar articles
-
Autocrine actions of prolactin contribute to the regulation of lactotroph function in vivo.FASEB J. 2018 Sep;32(9):4791-4797. doi: 10.1096/fj.201701111RR. Epub 2018 Mar 29. FASEB J. 2018. PMID: 29596024
-
Reversible transdifferentiation: interconversion of somatotrophs and lactotrophs in pituitary hyperplasia.Mod Pathol. 2001 Jan;14(1):20-8. doi: 10.1038/modpathol.3880252. Mod Pathol. 2001. PMID: 11211306
-
Increased lactotrophs despite decreased somatotrophs in the dwarf (dw/dw) rat: a defect in the regulation of lactotroph/somatotroph cell fate?J Endocrinol. 2002 Nov;175(2):435-46. doi: 10.1677/joe.0.1750435. J Endocrinol. 2002. PMID: 12429041
-
Plasticity of the prolactin (PRL) axis: mechanisms underlying regulation of output in female mice.Adv Exp Med Biol. 2015;846:139-62. doi: 10.1007/978-3-319-12114-7_6. Adv Exp Med Biol. 2015. PMID: 25472537 Review.
-
Prolactin and Growth Hormone Signaling and Interlink Focused on the Mammosomatotroph Paradigm: A Comprehensive Review of the Literature.Int J Mol Sci. 2023 Sep 12;24(18):14002. doi: 10.3390/ijms241814002. Int J Mol Sci. 2023. PMID: 37762304 Free PMC article. Review.
Cited by
-
Pituitary stem cells: past, present and future perspectives.Nat Rev Endocrinol. 2024 Feb;20(2):77-92. doi: 10.1038/s41574-023-00922-4. Epub 2023 Dec 15. Nat Rev Endocrinol. 2024. PMID: 38102391 Free PMC article. Review.
-
A New Perspective on Regulation of Pituitary Plasticity: The Network of SOX2-Positive Cells May Coordinate Responses to Challenge.Endocrinology. 2022 Aug 1;163(8):bqac089. doi: 10.1210/endocr/bqac089. Endocrinology. 2022. PMID: 35713880 Free PMC article.
-
Dose-dependent dual role of PIT-1 (POU1F1) in somatolactotroph cell proliferation and apoptosis.PLoS One. 2015 Mar 30;10(3):e0120010. doi: 10.1371/journal.pone.0120010. eCollection 2015. PLoS One. 2015. PMID: 25822178 Free PMC article.
-
The Transcription Factor NR4A2 Plays an Essential Role in Driving Prolactin Expression in Female Pituitary Lactotropes.Endocrinology. 2020 May 1;161(5):bqaa046. doi: 10.1210/endocr/bqaa046. Endocrinology. 2020. PMID: 32188976 Free PMC article.
-
Estrogens induce expression of membrane-associated estrogen receptor α isoforms in lactotropes.PLoS One. 2012;7(7):e41299. doi: 10.1371/journal.pone.0041299. Epub 2012 Jul 23. PLoS One. 2012. PMID: 22844453 Free PMC article.
References
-
- Ahlbom E, Grandison L, Zhivotovsky B, Ceccatelli S. Termination of lactation induces apoptosis and alters the expression of the Bcl-2 family members in the rat anterior pituitary. Endocrinology. 1998;139:2465–2471. - PubMed
-
- Aoki A, de Gaisan EO, Pasolli HA, Torres AL. Disposal of cell debris from surplus lactotrophs of pituitary gland. Experimental and Clinical Endocrinology and Diabetes. 1996;104:256–262. - PubMed
-
- Asa SL, Penz G, Kovacs K, Ezrin C. Prolactin cells in the human pituitary. A quantitative immunocytochemical analysis. Archives of Pathology & Laboratory Medicine. 1982;106:360–363. - PubMed
-
- Behringer RR, Mathews LS, Palmiter RD, Brinster RE. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes and Development. 1988;2:453–461. - PubMed
-
- Borrelli E, Heyman RA, Arias C, Sawchenko PE, Evans RM. Transgenic mice with inducible dwarfism. Nature. 1989;339:538–541. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

