Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response
- PMID: 20139186
- PMCID: PMC2838041
- DOI: 10.1128/JB.00988-09
Genetic modification of the Salmonella membrane physical state alters the pattern of heat shock response
Abstract
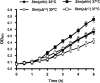
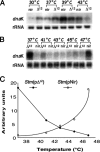
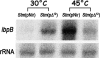
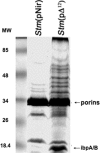
It is now recognized that membranes are not simple physical barriers but represent a complex and dynamic environment that affects membrane protein structures and their functions. Recent data emphasize the role of membranes in sensing temperature changes, and it has been shown that the physical state of the plasma membrane influences the expression of a variety of genes such as heat shock genes. It has been widely shown that minor alterations in lipid membranes are critically involved in the conversion of signals from the environment to the transcriptional activation of heat shock genes. Previously, we have proposed that the composition, molecular arrangement, and physical state of lipid membranes and their organization have crucial roles in cellular responses during stress caused by physical and chemical factors as well as in pathological states. Here, we show that transformation of Salmonella enterica serovar Typhimurium LT2 (Salmonella Typhimurium) with a heterologous Delta(12)-desaturase (or with its trans-membrane regions) causes major changes in the pathogen's membrane dynamic. In addition, this pathogen is strongly impaired in the synthesis of major stress proteins (heat shock proteins) under heat shock. These data support the hypothesis that the perception of temperature in Salmonella is strictly controlled by membrane order and by a specific membrane lipid/protein ratio that ultimately causes transcriptional activation of heat shock genes. These results represent a previously unrecognized mode of sensing temperature variation used by this pathogen at the onset of infection.
Figures


Similar articles
-
Changes in membrane fluid state and heat shock response cause attenuation of virulence.J Bacteriol. 2010 Apr;192(7):1999-2005. doi: 10.1128/JB.00990-09. Epub 2010 Feb 5. J Bacteriol. 2010. PMID: 20139193 Free PMC article.
-
Insertion of a 59 amino acid peptide in Salmonella Typhimurium membrane results in loss of virulence in mice.FEBS J. 2014 Nov;281(22):5043-53. doi: 10.1111/febs.13042. Epub 2014 Oct 4. FEBS J. 2014. PMID: 25208333
-
The DnaK/DnaJ chaperone machinery of Salmonella enterica serovar Typhimurium is essential for invasion of epithelial cells and survival within macrophages, leading to systemic infection.Infect Immun. 2004 Mar;72(3):1364-73. doi: 10.1128/IAI.72.3.1364-1373.2004. Infect Immun. 2004. PMID: 14977940 Free PMC article.
-
Plasma membranes as heat stress sensors: from lipid-controlled molecular switches to therapeutic applications.Biochim Biophys Acta. 2014 Jun;1838(6):1594-618. doi: 10.1016/j.bbamem.2013.12.015. Epub 2013 Dec 27. Biochim Biophys Acta. 2014. PMID: 24374314 Review.
-
[Regulation of heat shock gene expression in response to stress].Mol Biol (Mosk). 2017 May-Jun;51(3):400-417. doi: 10.7868/S0026898417020100. Mol Biol (Mosk). 2017. PMID: 28707656 Review. Russian.
Cited by
-
Growth-Environment Dependent Modulation of Staphylococcus aureus Branched-Chain to Straight-Chain Fatty Acid Ratio and Incorporation of Unsaturated Fatty Acids.PLoS One. 2016 Oct 27;11(10):e0165300. doi: 10.1371/journal.pone.0165300. eCollection 2016. PLoS One. 2016. PMID: 27788193 Free PMC article.
-
Branched-chain fatty acids promote Listeria monocytogenes intracellular infection and virulence.Infect Immun. 2010 Nov;78(11):4667-73. doi: 10.1128/IAI.00546-10. Epub 2010 Sep 7. Infect Immun. 2010. PMID: 20823206 Free PMC article.
-
Immediate reduction of Salmonella enterica serotype typhimurium viability via membrane destabilization following exposure to multiple-hurdle treatments with heated, acidified organic acid salt solutions.Appl Environ Microbiol. 2011 Jun;77(11):3765-72. doi: 10.1128/AEM.02839-10. Epub 2011 Apr 8. Appl Environ Microbiol. 2011. PMID: 21478311 Free PMC article.
-
Changes in membrane fluid state and heat shock response cause attenuation of virulence.J Bacteriol. 2010 Apr;192(7):1999-2005. doi: 10.1128/JB.00990-09. Epub 2010 Feb 5. J Bacteriol. 2010. PMID: 20139193 Free PMC article.
-
Regulatory role of membrane fluidity in gene expression and physiological functions.Photosynth Res. 2013 Oct;116(2-3):489-509. doi: 10.1007/s11120-013-9823-4. Epub 2013 Apr 20. Photosynth Res. 2013. PMID: 23605242 Review.
References
-
- Alvarez-Ordóñez, A., A. Fernández, M. López, R. Arenas, and A. Bernardo. 2008. Modifications in membrane fatty acid composition of Salmonella typhimurium in response to growth conditions and their effect on heat resistance. Int. J. Food Microbiol. 123:212-219. - PubMed
-
- Balogh, G., I. Horváth, E. Nagy, Z. Hoyk, S. Benkõ, and L. Vígh. 2005. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 272:6077-6086. - PubMed
-
- Beere, H. M. 2004. “The stress of dying”: the role of heat shock proteins in the regulation of apoptosis. J. Cell Sci. 117:2641-2651. - PubMed
-
- Broadley, S. A., and F. U. Hartl. 2008. Mitochondrial stress signaling: a pathway unfolds. Trends Cell Biol. 18:1-4. - PubMed
-
- Broquet, A. H., G. Thomas, J. Masliah, G. Trugnan, and M. Bachelet. 2003. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J. Biol. Chem. 278:21601-21606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

