Genetic engineering of algae for enhanced biofuel production
- PMID: 20139239
- PMCID: PMC2863401
- DOI: 10.1128/EC.00364-09
Genetic engineering of algae for enhanced biofuel production
Abstract
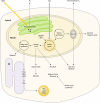
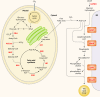
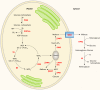
There are currently intensive global research efforts aimed at increasing and modifying the accumulation of lipids, alcohols, hydrocarbons, polysaccharides, and other energy storage compounds in photosynthetic organisms, yeast, and bacteria through genetic engineering. Many improvements have been realized, including increased lipid and carbohydrate production, improved H(2) yields, and the diversion of central metabolic intermediates into fungible biofuels. Photosynthetic microorganisms are attracting considerable interest within these efforts due to their relatively high photosynthetic conversion efficiencies, diverse metabolic capabilities, superior growth rates, and ability to store or secrete energy-rich hydrocarbons. Relative to cyanobacteria, eukaryotic microalgae possess several unique metabolic attributes of relevance to biofuel production, including the accumulation of significant quantities of triacylglycerol; the synthesis of storage starch (amylopectin and amylose), which is similar to that found in higher plants; and the ability to efficiently couple photosynthetic electron transport to H(2) production. Although the application of genetic engineering to improve energy production phenotypes in eukaryotic microalgae is in its infancy, significant advances in the development of genetic manipulation tools have recently been achieved with microalgal model systems and are being used to manipulate central carbon metabolism in these organisms. It is likely that many of these advances can be extended to industrially relevant organisms. This review is focused on potential avenues of genetic engineering that may be undertaken in order to improve microalgae as a biofuel platform for the production of biohydrogen, starch-derived alcohols, diesel fuel surrogates, and/or alkanes.
Figures
References
-
- Apt K. E., Grossman A. R., Kroth-Pancic P. G. 1996. Stable nuclear transformation of the diatom Phaeodactylum tricornutum. Mol. Gen. Genet. 252:572–579 - PubMed
-
- Apt K. E., Zaslavkaia L., Lippmeier J. C., Lang M., Kilian O., Wetherbee R., Grossman A. R., Kroth P. G. 2002. In vivo characterization of diatom multipartite plastid targeting signals. J. Cell Sci. 115:4061–4069 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

