K(V)4.2 channels tagged in the S1-S2 loop for alpha-bungarotoxin binding provide a new tool for studies of channel expression and localization
- PMID: 20139708
- PMCID: PMC2888848
- DOI: 10.4161/chan.4.2.10878
K(V)4.2 channels tagged in the S1-S2 loop for alpha-bungarotoxin binding provide a new tool for studies of channel expression and localization
Abstract
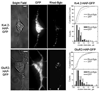
We report the first successful insertion of an engineered, high-affinity alpha-bungarotoxin (Bgtx) binding site into a voltage-gated ion channel, K(V)4.2, using a short, intra-protein embedded sequence (GGWRYYESSLEPYPDGG), derived from a previously described mimotope peptide, HAP. A major benefit to this approach is the ability to live-image the distribution and fate of functional channels on the plasma membrane surface. The Bgtx binding sequence was introduced into the putative extracellular loop between the S1 and S2 transmembrane domains of K(V)4.2. Following co-expression with KChIP3 in tsA201 cells, S1-S2 HAP-tagged channels express at levels comparable to wild-type K(V)4.2, and their activation and inactivation kinetics are minimally altered under most conditions. Binding assays, as well as live staining of surface-expressed K(V)4.2 channels with fluorescent-Bgtx, readily demonstrate specific binding of Bgtx to HAP-tagged K(V)4.2 expressed on the surface of tsA201 cells. Similar live-imaging results were obtained with HAP-tagged K(V)4.2 transfected into hippocampal neurons in primary culture suggesting applicability for future in vivo studies. Furthermore, the activation kinetics of S1-S2-tagged K(V)4.2 channels are minimally affected by the binding of Bgtx, suggesting a limited role if any for the S1-S2 loop in voltage sensing or gating associated conformational changes. Successful functional insertion of the HAP sequence into the S1-S2 linker of K(V)4.2 suggests that other related channels may similarly be amenable to this tagging strategy.
Conflict of interest statement
Figures


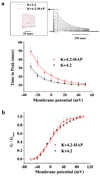
 ). (a) Time to peak current analysis. Representative current traces of inactivation for Kv4.2 and Kv4.2-HAP are shown in Fig1(a). The upper panel shows detailed superimposed peak currents for Kv4.2 (black) and Kv4.2-HAP (red) in response to step membrane depolarization. Student’s t test analysis showed that for voltage steps +20 mV to +50 mV the mean times to peak for Kv4.2 and Kv4.2-HAP were not significantly different (p > 0.05). In contrast, for the −30 to +10 mV range, the results are significantly different (p < 0.05). (b) Normalized peak conductance-voltage relations for Kv4.2 and Kv4.2-HAP. Normalized conductance (G/Gmax, conductances at the indicated potential divided by Gmax, the maximum conductance, at +50 mV) plotted as a function of voltage for the currents expressed by Kv4.2 and Kv4.2-HAP. Peak conductance (G) was calculated as G = Ip/(Vm − Veq), where Ip, Vm and Veq are the peak current, the test potential and the K+ equilibrium potential, respectively. The continuous lines across the data points are the best-fits to Boltzmann functions. Values represent the Mean ± S.E.M of three to four cells. Normalized peak conductance-voltage relations for Kv4.2 and Kv4.2-HAP at all voltage steps are not significantly different by the Student’s t test (p > 0.05).
). (a) Time to peak current analysis. Representative current traces of inactivation for Kv4.2 and Kv4.2-HAP are shown in Fig1(a). The upper panel shows detailed superimposed peak currents for Kv4.2 (black) and Kv4.2-HAP (red) in response to step membrane depolarization. Student’s t test analysis showed that for voltage steps +20 mV to +50 mV the mean times to peak for Kv4.2 and Kv4.2-HAP were not significantly different (p > 0.05). In contrast, for the −30 to +10 mV range, the results are significantly different (p < 0.05). (b) Normalized peak conductance-voltage relations for Kv4.2 and Kv4.2-HAP. Normalized conductance (G/Gmax, conductances at the indicated potential divided by Gmax, the maximum conductance, at +50 mV) plotted as a function of voltage for the currents expressed by Kv4.2 and Kv4.2-HAP. Peak conductance (G) was calculated as G = Ip/(Vm − Veq), where Ip, Vm and Veq are the peak current, the test potential and the K+ equilibrium potential, respectively. The continuous lines across the data points are the best-fits to Boltzmann functions. Values represent the Mean ± S.E.M of three to four cells. Normalized peak conductance-voltage relations for Kv4.2 and Kv4.2-HAP at all voltage steps are not significantly different by the Student’s t test (p > 0.05).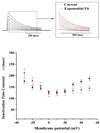
 ). The upper panel shows inactivation kinetics of currents in response to step membrane depolarization. The time course of fast inactivation fits mono-exponentially. The continuous lines in red overlaid represent single exponential fits. Note that, the rate of inactivation increases with strong depolarization. Rate of inactivation decreased in the voltage range from −30 mV to 0 mV and increased from 0 mV to +50 mV. Values represent the Mean ± S.E.M of three to four cells. Student’s t test analysis showed that the means of inactivation time constants τ for Kv4.2 and Kv4.2-HAP at voltage steps ranging from −20 mV to +20 mV are not significantly different (p > 0.05). In contrast, they are significantly different at voltage steps of −30 mV, +30 mV, +40 mV and +50 mV (p < 0.05).
). The upper panel shows inactivation kinetics of currents in response to step membrane depolarization. The time course of fast inactivation fits mono-exponentially. The continuous lines in red overlaid represent single exponential fits. Note that, the rate of inactivation increases with strong depolarization. Rate of inactivation decreased in the voltage range from −30 mV to 0 mV and increased from 0 mV to +50 mV. Values represent the Mean ± S.E.M of three to four cells. Student’s t test analysis showed that the means of inactivation time constants τ for Kv4.2 and Kv4.2-HAP at voltage steps ranging from −20 mV to +20 mV are not significantly different (p > 0.05). In contrast, they are significantly different at voltage steps of −30 mV, +30 mV, +40 mV and +50 mV (p < 0.05).
 ) recovers slightly more slowly from inactivation than wild-type Kv4.2 (■). Values represent the Mean ± S.E.M of data obtained from three to four cells. Applying the Student’s t test, the rate of recovery from inactivation for Kv4.2-HAP is not significantly different (p > 0.05) from that of Kv4.2.
) recovers slightly more slowly from inactivation than wild-type Kv4.2 (■). Values represent the Mean ± S.E.M of data obtained from three to four cells. Applying the Student’s t test, the rate of recovery from inactivation for Kv4.2-HAP is not significantly different (p > 0.05) from that of Kv4.2.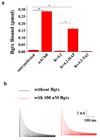

Similar articles
-
Somatodendritic surface expression of epitope-tagged and KChIP binding-deficient Kv4.2 channels in hippocampal neurons.PLoS One. 2018 Jan 31;13(1):e0191911. doi: 10.1371/journal.pone.0191911. eCollection 2018. PLoS One. 2018. PMID: 29385176 Free PMC article.
-
A novel bungarotoxin binding site-tagged construct reveals MAPK-dependent Kv4.2 trafficking.Mol Cell Neurosci. 2019 Jul;98:121-130. doi: 10.1016/j.mcn.2019.06.007. Epub 2019 Jun 15. Mol Cell Neurosci. 2019. PMID: 31212013 Free PMC article.
-
Contribution of N- and C-terminal Kv4.2 channel domains to KChIP interaction [corrected].J Physiol. 2005 Oct 15;568(Pt 2):397-412. doi: 10.1113/jphysiol.2005.094359. Epub 2005 Aug 11. J Physiol. 2005. PMID: 16096338 Free PMC article.
-
Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs.Int J Mol Sci. 2021 Jan 31;22(3):1419. doi: 10.3390/ijms22031419. Int J Mol Sci. 2021. PMID: 33572566 Free PMC article. Review.
-
The neuronal Kv4 channel complex.Neurochem Res. 2008 Aug;33(8):1558-67. doi: 10.1007/s11064-008-9650-8. Epub 2008 Mar 21. Neurochem Res. 2008. PMID: 18357523 Free PMC article. Review.
Cited by
-
GABAA receptor membrane insertion rates are specified by their subunit composition.Mol Cell Neurosci. 2013 Sep;56:201-11. doi: 10.1016/j.mcn.2013.05.003. Epub 2013 May 25. Mol Cell Neurosci. 2013. PMID: 23714576 Free PMC article.
-
Snake neurotoxin α-bungarotoxin is an antagonist at native GABA(A) receptors.Neuropharmacology. 2015 Jun;93:28-40. doi: 10.1016/j.neuropharm.2015.01.001. Epub 2015 Jan 26. Neuropharmacology. 2015. PMID: 25634239 Free PMC article.
-
Plasma membrane insertion of epithelial sodium channels occurs with dual kinetics.Pflugers Arch. 2016 May;468(5):859-70. doi: 10.1007/s00424-016-1799-4. Epub 2016 Feb 15. Pflugers Arch. 2016. PMID: 26876388
-
Somatodendritic surface expression of epitope-tagged and KChIP binding-deficient Kv4.2 channels in hippocampal neurons.PLoS One. 2018 Jan 31;13(1):e0191911. doi: 10.1371/journal.pone.0191911. eCollection 2018. PLoS One. 2018. PMID: 29385176 Free PMC article.
-
Design of Ultrapotent Genetically Encoded Inhibitors of Kv4.2 for Gating Neural Plasticity.J Neurosci. 2024 Feb 14;44(7):e2295222023. doi: 10.1523/JNEUROSCI.2295-22.2023. J Neurosci. 2024. PMID: 38154956 Free PMC article.
References
-
- Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, et al. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. - PubMed
-
- Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, et al. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. - PubMed
-
- Bahring R, Dannenberg J, Peters HC, Leicher T, Pongs O, Isbrandt D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J Biol Chem. 2001;276:23888–23894. - PubMed
-
- Heusser K, Schwappach B. Trafficking of potassium channels. Curr Opin Neurobiol. 2005;15:364–369. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
