Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses
- PMID: 20139771
- PMCID: PMC2947860
- DOI: 10.1097/HJH.0b013e328336ed3e
Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses
Abstract
Objective: The phenotype of the chromogranin A (Chga) null (knockout) mouse is hypertensive. However, hypertensive humans and spontaneously hypertensive rats display elevated CHGA expression. This study addresses the paradox that both ablation and elevation of CHGA result in hypertension.
Methods: Mice with varying copy number of the CHGA gene were generated. In these mice CHGA, catecholamine and blood pressure (BP) were measured. Also a cohort of healthy human individuals was stratified into tertiles based on plasma CHGA expression and phenotyped for characteristics including their BP response to environmental (cold) stress.
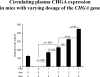
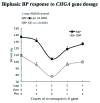
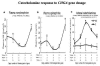
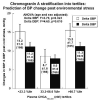
Results: The mice displayed a direct CHGA gene dose-dependent (0-4 copies/genome) activation of CHGA expression in both plasma and adrenal gland, yet the BP dependence of CHGA gene dose was U-shaped, maximal at 0 and four copies of the gene, whereas minimal at two copies (i.e., the wild-type gene dosage). Plasma catecholamine showed a parallel U-shaped dose/response in mice, whereas adrenal epinephrine exhibited a reciprocal (inverted) U-shaped response, suggesting dysregulated neurotransmission at both extremes of CHGA expression. The human individuals also showed a nonlinear relationship between CHGA expression and pressor responses to environmental (cold) stress, that were maximal in the highest and lowest tertiles, though basal BPs did not differ among the groups. The human CHGA tertiles also differed in epinephrine secretion as well as degree of CHGA processing to catestatin (catecholamine release-inhibitory peptide derived from CHGA processing).
Conclusion: Thus, across mammalian species, an optimal amount of CHGA may be required to establish appropriate catecholamine storage and release, and hence BP homeostasis.
Conflict of interest statement
Figures


Comment in
-
Indices of sympathetic activity and the paradox of chromogranin A.J Hypertens. 2010 Apr;28(4):676-8. doi: 10.1097/HJH.0b013e32833857e3. J Hypertens. 2010. PMID: 20625251 No abstract available.
Similar articles
-
Urocortin 2 lowers blood pressure and reduces plasma catecholamine levels in mice with hyperadrenergic activity.Endocrinology. 2010 Oct;151(10):4820-9. doi: 10.1210/en.2009-1454. Epub 2010 Jul 28. Endocrinology. 2010. PMID: 20668031 Free PMC article.
-
Long human CHGA flanking chromosome 14 sequence required for optimal BAC transgenic "rescue" of disease phenotypes in the mouse Chga knockout.Physiol Genomics. 2010 Mar 3;41(1):91-101. doi: 10.1152/physiolgenomics.00086.2009. Epub 2009 Dec 15. Physiol Genomics. 2010. PMID: 20009010 Free PMC article.
-
Mice overexpressing chromogranin A display hypergranulogenic adrenal glands with attenuated ATP levels contributing to the hypertensive phenotype.J Hypertens. 2018 May;36(5):1115-1128. doi: 10.1097/HJH.0000000000001678. J Hypertens. 2018. PMID: 29389743 Free PMC article.
-
Chromogranin A: a surprising link between granule biogenesis and hypertension.J Clin Invest. 2005 Jul;115(7):1711-3. doi: 10.1172/JCI25706. J Clin Invest. 2005. PMID: 16007250 Free PMC article. Review.
-
Chromogranin A: a novel susceptibility gene for essential hypertension.Cell Mol Life Sci. 2010 Mar;67(6):861-74. doi: 10.1007/s00018-009-0208-y. Epub 2009 Nov 27. Cell Mol Life Sci. 2010. PMID: 19943077 Free PMC article. Review.
Cited by
-
Catestatin reverses the hypertrophic effects of norepinephrine in H9c2 cardiac myoblasts by modulating the adrenergic signaling.Mol Cell Biochem. 2020 Jan;464(1-2):205-219. doi: 10.1007/s11010-019-03661-1. Epub 2019 Dec 2. Mol Cell Biochem. 2020. PMID: 31792650
-
Prevention of vascular calcification by the endogenous chromogranin A-derived mediator that inhibits osteogenic transdifferentiation.Basic Res Cardiol. 2021 Oct 13;116(1):57. doi: 10.1007/s00395-021-00899-z. Basic Res Cardiol. 2021. PMID: 34647168 Free PMC article.
-
Disorders of blood pressure regulation-role of catecholamine biosynthesis, release, and metabolism.Curr Hypertens Rep. 2012 Feb;14(1):38-45. doi: 10.1007/s11906-011-0239-2. Curr Hypertens Rep. 2012. PMID: 22068338 Review.
-
The Influence of the Sympathetic Nervous System on Cardiometabolic Health in Response to Weight Gain or Weight Loss.Metabolites. 2025 Apr 23;15(5):286. doi: 10.3390/metabo15050286. Metabolites. 2025. PMID: 40422864 Free PMC article. Review.
-
Cysteine Cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer's disease.Biochim Biophys Acta. 2012 Jan;1824(1):89-104. doi: 10.1016/j.bbapap.2011.08.015. Epub 2011 Sep 8. Biochim Biophys Acta. 2012. PMID: 21925292 Free PMC article. Review.
References
-
- O'Connor DT, Frigon RP. Chromogranin A, the major catecholamine storage vesicle soluble protein. Multiple size forms, subcellular storage, and regional distribution in chromaffin and nervous tissue elucidated by radioimmunoassay. J Biol Chem. 1984;259:3237–3247. - PubMed
-
- Taupenot L, Harper KL, O'Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. - PubMed
-
- O'Connor DT. Chromogranin A: implications for hypertension. J Hypertens Suppl. 1984;2:S147–150. - PubMed
-
- Takiyyuddin MA, Cervenka JH, Hsiao RJ, Barbosa JA, Parmer RJ, O'Connor DT. Chromogranin A. Storage and release in hypertension Hypertension. 1990;15:237–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

