Tbx3 improves the germ-line competency of induced pluripotent stem cells
- PMID: 20139965
- PMCID: PMC2901797
- DOI: 10.1038/nature08735
Tbx3 improves the germ-line competency of induced pluripotent stem cells
Abstract
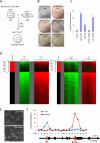
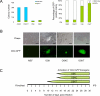
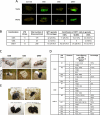
Induced pluripotent stem (iPS) cells can be obtained by the introduction of defined factors into somatic cells. The combination of Oct4 (also known as Pou5f1), Sox2 and Klf4 (which we term OSK) constitutes the minimal requirement for generating iPS cells from mouse embryonic fibroblasts. These cells are thought to resemble embryonic stem cells (ESCs) on the basis of global gene expression analyses; however, few studies have tested the ability and efficiency of iPS cells to contribute to chimaerism, colonization of germ tissues, and most importantly, germ-line transmission and live birth from iPS cells produced by tetraploid complementation. Using genomic analyses of ESC genes that have roles in pluripotency and fusion-mediated somatic cell reprogramming, here we show that the transcription factor Tbx3 significantly improves the quality of iPS cells. iPS cells generated with OSK and Tbx3 (OSKT) are superior in both germ-cell contribution to the gonads and germ-line transmission frequency. However, global gene expression profiling could not distinguish between OSK and OSKT iPS cells. Genome-wide chromatin immunoprecipitation sequencing analysis of Tbx3-binding sites in ESCs suggests that Tbx3 regulates pluripotency-associated and reprogramming factors, in addition to sharing many common downstream regulatory targets with Oct4, Sox2, Nanog and Smad1. This study underscores the intrinsic qualitative differences between iPS cells generated by different methods, and highlights the need to rigorously characterize iPS cells beyond in vitro studies.
Figures

References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Shi Y, et al. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–74. - PubMed
-
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

