Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae
- PMID: 20139971
- PMCID: PMC2856316
- DOI: 10.1038/ncb2030
Histone H3 Thr 45 phosphorylation is a replication-associated post-translational modification in S. cerevisiae
Abstract
Post-translational histone modifications are crucial for the regulation of numerous DNA-templated processes, and are thought to mediate both alteration of chromatin dynamics and recruitment of effector proteins to specific regions of the genome. In particular, histone Ser/Thr phosphorylation regulates multiple nuclear functions in the budding yeast Saccharomyces cerevisiae, including transcription, DNA damage repair, mitosis, apoptosis and sporulation. Although modifications to chromatin during replication remain poorly understood, a number of recent studies have described acetylation of the histone H3 N-terminal alpha-helix (alphaN helix) at Lys 56 as a modification that is important for maintenance of genomic integrity during DNA replication and repair. Here, we report phosphorylation of H3 Thr 45 (H3-T45), a histone modification also located within the H3 alphaN helix in S. cerevisiae. Thr 45 phosphorylation peaks during DNA replication, and is mediated by the S phase kinase Cdc7-Dbf4 as part of a multiprotein complex identified in this study. Furthermore, loss of phosphorylated H3-T45 causes phenotypes consistent with replicative defects, and prolonged replication stress results in H3-T45 phosphorylation accumulation over time. Notably, the phenotypes described here are independent of Lys 56 acetylation status, and combinatorial mutations to both Thr 45 and Lys 56 of H3 cause synthetic growth defects. Together, these data identify and characterize H3-T45 phosphorylation as a replication-associated histone modification in budding yeast.
Figures

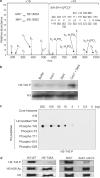
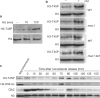
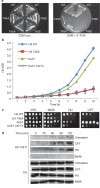
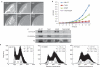
References
-
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. - PubMed
-
- Ito T. Role of histone modification in chromatin dynamics. J. Biochem. 2007;141:609–614. - PubMed
-
- Masumoto H, Hawke D, Kobayashi R, Verreault A. A role for cell-cycle-regulated histone H3 lysine 56 acetylation in the DNA damage response. Nature. 2005;436:294–298. - PubMed
-
- Han J, et al. Rtt109 acetylates histone H3 lysine 56 and functions in DNA replication. Science. 2007;315:653–655. - PubMed
-
- Grant PA, Berger SL, Workman JL. Identification and analysis of native nucleosomal histone acetyltransferase complexes. Methods Mol. Biol. 1999;119:311–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 5 P30 CA044579-18/CA/NCI NIH HHS/United States
- R01 GM060444/GM/NIGMS NIH HHS/United States
- GM60444/GM/NIGMS NIH HHS/United States
- 5 T32 CA009109-30/CA/NCI NIH HHS/United States
- P30 CA044579/CA/NCI NIH HHS/United States
- R01 GM037537/GM/NIGMS NIH HHS/United States
- R56 DK082673-01/DK/NIDDK NIH HHS/United States
- T32 CA009109/CA/NCI NIH HHS/United States
- GM37537/GM/NIGMS NIH HHS/United States
- R56 DK082673/DK/NIDDK NIH HHS/United States
- R56 GM060444/GM/NIGMS NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

