Differential binding of bispyridinium oxime drugs with acetylcholinesterase
- PMID: 20140002
- PMCID: PMC4002407
- DOI: 10.1038/aps.2009.193
Differential binding of bispyridinium oxime drugs with acetylcholinesterase
Abstract
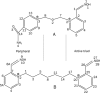
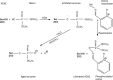
Aim: To performe a time-dependent topographical delineation of protein-drug interactions to gain molecular insight into the supremacy of Ortho-7 over HI-6 in reactivating tabun-conjugated mouse acetylcholinesterase (mAChE).
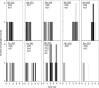
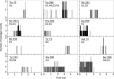
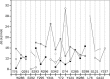
Methods: We conducted all-atom steered molecular dynamics simulations of the two protein-drug complexes. Through a host of protein-drug interaction parameters (rupture force profiles, hydrogen bonds, water bridges, hydrophobic interactions), geometrical, and orientation ordering of the drugs, we monitored the enzyme's response during the release of the drugs from its active-site.
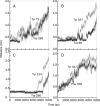
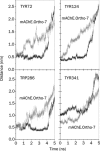
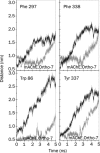
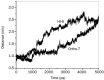
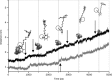
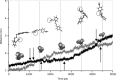
Results: The results show the preferential binding of the drugs with the enzyme. The pyridinium ring of HI-6 shows excellent complementary binding with the peripheral anionic site, whereas one of two identical pyridinium rings of Ortho-7 has excellent binding compatibility in the enzyme active-site where it can orchestrate the reactivation process. We found that the active pyridinium ring of HI-6 undergoes a complete turn along the active site axis, directed away from the active-site region during the course of the simulation.
Conclusion: Due to excellent cooperative binding of Ortho-7, as rendered by several cation-pi interactions with the active-site gorge of the enzyme, Ortho-7 may be a more efficient reactivator than HI-6. Our work supports the growing body of evidence that the efficacy of the drugs is due to the differential bindings of the oximes with AChE and can aid to the rational design of oxime drugs.
Figures




Similar articles
-
In silico studies on the role of mutant Y337A to reactivate tabun inhibited mAChE with K048.Chem Biol Interact. 2015 Dec 5;242:299-306. doi: 10.1016/j.cbi.2015.10.016. Epub 2015 Oct 19. Chem Biol Interact. 2015. PMID: 26494532
-
Can hydroxylamine be a more potent nucleophile for the reactivation of tabun-inhibited AChE than prototype oxime drugs? An answer derived from quantum chemical and steered molecular dynamics studies.Mol Biosyst. 2014 Jul 29;10(9):2368-83. doi: 10.1039/c4mb00083h. Mol Biosyst. 2014. PMID: 24964273
-
Crystal structures of acetylcholinesterase in complex with HI-6, Ortho-7 and obidoxime: structural basis for differences in the ability to reactivate tabun conjugates.Biochem Pharmacol. 2006 Aug 28;72(5):597-607. doi: 10.1016/j.bcp.2006.05.027. Epub 2006 Jul 31. Biochem Pharmacol. 2006. PMID: 16876764
-
The reactivation of tabun-inhibited mutant AChE with Ortho-7: steered molecular dynamics and quantum chemical studies.Mol Biosyst. 2016 Apr;12(4):1224-31. doi: 10.1039/c5mb00735f. Epub 2016 Feb 16. Mol Biosyst. 2016. PMID: 26879641
-
Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds.Curr Med Chem. 2009;16(17):2177-88. doi: 10.2174/092986709788612729. Curr Med Chem. 2009. PMID: 19519385 Review.
Cited by
-
Energetics of Ortho-7 (oxime drug) translocation through the active-site gorge of tabun conjugated acetylcholinesterase.PLoS One. 2012;7(7):e40188. doi: 10.1371/journal.pone.0040188. Epub 2012 Jul 11. PLoS One. 2012. PMID: 22808117 Free PMC article.
-
Influence of gauche effect on uncharged oxime reactivators for the reactivation of tabun-inhibited AChE: quantum chemical and steered molecular dynamics studies.J Comput Aided Mol Des. 2018 Jul;32(7):793-807. doi: 10.1007/s10822-018-0130-1. Epub 2018 Jul 6. J Comput Aided Mol Des. 2018. PMID: 29980922
-
Revealing the importance of linkers in K-series oxime reactivators for tabun-inhibited AChE using quantum chemical, docking and SMD studies.J Comput Aided Mol Des. 2017 Aug;31(8):729-742. doi: 10.1007/s10822-017-0036-3. Epub 2017 Jun 23. J Comput Aided Mol Des. 2017. PMID: 28646405
-
FDA-Approved Oximes and Their Significance in Medicinal Chemistry.Pharmaceuticals (Basel). 2022 Jan 4;15(1):66. doi: 10.3390/ph15010066. Pharmaceuticals (Basel). 2022. PMID: 35056123 Free PMC article. Review.
-
Functional Analysis and Molecular Docking studies of Medicinal Compounds for AChE and BChE in Alzheimer's Disease and Type 2 Diabetes Mellitus.Aging Dis. 2013 Jun 20;4(4):186-200. Print 2013 Aug. Aging Dis. 2013. PMID: 23936743 Free PMC article.
References
-
- Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states. Chem Rev. 1987;87:955–79.
-
- Kandel ER, Schwartz JH, Jessell TM.Principles of neural science. 3rd ed. Norwalk, CT: Appleton & Lange; 1991
-
- Barnard EA.In the peripheral nervous system. Hubbard JI, Editor. New York: Plenum Publishers; 1974. p201–6.
-
- Voet D, Voet JG.Biochemistry. 2nd edition. Wiley & Sons; 1995
-
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–58. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

