Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma
- PMID: 20140016
- PMCID: PMC2861731
- DOI: 10.1038/onc.2010.10
Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma
Abstract
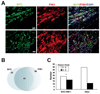
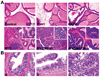
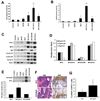
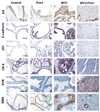
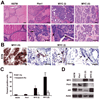
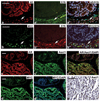
The oncogenic PIM1 kinase has been implicated as a cofactor for c-MYC in prostate carcinogenesis. In this study, we show that in human prostate tumors, coexpression of c-MYC and PIM1 is associated with higher Gleason grades. Using a tissue recombination model coupled with lentiviral-mediated gene transfer we find that Pim1 is weakly oncogenic in naive adult mouse prostatic epithelium. However, it cooperates dramatically with c-MYC to induce prostate cancer within 6-weeks. Importantly, c-MYC/Pim1 synergy is critically dependent on Pim1 kinase activity. c-MYC/Pim1 tumors showed increased levels of the active serine-62 (S62) phosphorylated form of c-MYC. Grafts expressing a phosphomimetic c-MYCS62D mutant had higher rates of proliferation than grafts expressing wild type c-MYC but did not form tumors like c-MYC/Pim1 grafts, indicating that Pim1 cooperativity with c-MYC in vivo involves additional mechanisms other than enhancement of c-MYC activity by S62 phosphorylation. c-MYC/Pim1-induced prostate carcinomas show evidence of neuroendocrine (NE) differentiation. Additional studies, including the identification of tumor cells coexpressing androgen receptor and NE cell markers synaptophysin and Ascl1 suggested that NE tumors arose from adenocarcinoma cells through transdifferentiation. These results directly show functional cooperativity between c-MYC and PIM1 in prostate tumorigenesis in vivo and support efforts for targeting PIM1 in prostate cancer.
Figures

Similar articles
-
Pim1 promotes human prostate cancer cell tumorigenicity and c-MYC transcriptional activity.BMC Cancer. 2010 Jun 1;10:248. doi: 10.1186/1471-2407-10-248. BMC Cancer. 2010. PMID: 20515470 Free PMC article.
-
Pim1 kinase is required to maintain tumorigenicity in MYC-expressing prostate cancer cells.Oncogene. 2012 Apr 5;31(14):1794-803. doi: 10.1038/onc.2011.371. Epub 2011 Aug 22. Oncogene. 2012. PMID: 21860423 Free PMC article.
-
Kinase PIM1 promotes prostate cancer cell growth via c-Myc-RPS7-driven ribosomal stress.Carcinogenesis. 2019 Mar 12;40(1):52-60. doi: 10.1093/carcin/bgy126. Carcinogenesis. 2019. PMID: 30247545
-
PIM1 kinase as a target in prostate cancer: roles in tumorigenesis, castration resistance, and docetaxel resistance.Curr Cancer Drug Targets. 2014;14(2):105-14. doi: 10.2174/1568009613666131126113854. Curr Cancer Drug Targets. 2014. PMID: 24274399 Review.
-
Potential roles for the PIM1 kinase in human cancer - a molecular and therapeutic appraisal.Eur J Cancer. 2008 Oct;44(15):2144-51. doi: 10.1016/j.ejca.2008.06.044. Epub 2008 Aug 18. Eur J Cancer. 2008. PMID: 18715779 Review.
Cited by
-
The clinical and molecular characteristics of progressive hypothalamic/optic pathway pilocytic astrocytoma.Neuro Oncol. 2023 Apr 6;25(4):750-760. doi: 10.1093/neuonc/noac241. Neuro Oncol. 2023. PMID: 36260562 Free PMC article.
-
When Just One Phosphate Is One Too Many: The Multifaceted Interplay between Myc and Kinases.Int J Mol Sci. 2023 Mar 1;24(5):4746. doi: 10.3390/ijms24054746. Int J Mol Sci. 2023. PMID: 36902175 Free PMC article. Review.
-
The Pim protein kinases regulate energy metabolism and cell growth.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):528-33. doi: 10.1073/pnas.1013214108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187426 Free PMC article.
-
Cytoplasmic Irradiation Induces Metabolic Shift in Human Small Airway Epithelial Cells via Activation of Pim-1 Kinase.Radiat Res. 2017 Apr;187(4):441-453. doi: 10.1667/RR0006CC.1. Epub 2017 Feb 7. Radiat Res. 2017. PMID: 28170315 Free PMC article.
-
Loss of PIM2 enhances the anti-proliferative effect of the pan-PIM kinase inhibitor AZD1208 in non-Hodgkin lymphomas.Mol Cancer. 2015 Dec 8;14:205. doi: 10.1186/s12943-015-0477-z. Mol Cancer. 2015. PMID: 26643319 Free PMC article.
References
-
- Abdulkadir SA, Carbone JM, Naughton CK, Humphrey PA, Catalona WJ, Milbrandt J. Hum Pathol. 2001a;32:935–939. - PubMed
-
- Abdulkadir SA, Qu Z, Garabedian E, Song SK, Peters TJ, Svaren J, Carbone JM, Naughton CK, Catalona WJ, Ackerman JJ, Gordon JI, Humphrey PA, Milbrandt J. Nat Med. 2001b;7:101–107. - PubMed
-
- Aho TL, Sandholm J, Peltola KJ, Mankonen HP, Lilly M, Koskinen PJ. FEBS Lett. 2004;571:43–49. - PubMed
-
- Bachmann M, Hennemann H, Xing PX, Hoffmann I, Moroy T. J Biol Chem. 2004;279:48319–48328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

