How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer's beta amyloid peptide (Abeta1-40)
- PMID: 20140095
- PMCID: PMC2816720
- DOI: 10.1371/journal.pone.0009079
How cholesterol constrains glycolipid conformation for optimal recognition of Alzheimer's beta amyloid peptide (Abeta1-40)
Abstract
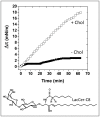
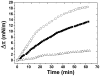
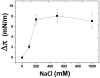
Membrane lipids play a pivotal role in the pathogenesis of Alzheimer's disease, which is associated with conformational changes, oligomerization and/or aggregation of Alzheimer's beta-amyloid (Abeta) peptides. Yet conflicting data have been reported on the respective effect of cholesterol and glycosphingolipids (GSLs) on the supramolecular assembly of Abeta peptides. The aim of the present study was to unravel the molecular mechanisms by which cholesterol modulates the interaction between Abeta(1-40) and chemically defined GSLs (GalCer, LacCer, GM1, GM3). Using the Langmuir monolayer technique, we show that Abeta(1-40) selectively binds to GSLs containing a 2-OH group in the acyl chain of the ceramide backbone (HFA-GSLs). In contrast, Abeta(1-40) did not interact with GSLs containing a nonhydroxylated fatty acid (NFA-GSLs). Cholesterol inhibited the interaction of Abeta(1-40) with HFA-GSLs, through dilution of the GSL in the monolayer, but rendered the initially inactive NFA-GSLs competent for Abeta(1-40) binding. Both crystallographic data and molecular dynamics simulations suggested that the active conformation of HFA-GSL involves a H-bond network that restricts the orientation of the sugar group of GSLs in a parallel orientation with respect to the membrane. This particular conformation is stabilized by the 2-OH group of the GSL. Correspondingly, the interaction of Abeta(1-40) with HFA-GSLs is strongly inhibited by NaF, an efficient competitor of H-bond formation. For NFA-GSLs, this is the OH group of cholesterol that constrains the glycolipid to adopt the active L-shape conformation compatible with sugar-aromatic CH-pi stacking interactions involving residue Y10 of Abeta(1-40). We conclude that cholesterol can either inhibit or facilitate membrane-Abeta interactions through fine tuning of glycosphingolipid conformation. These data shed some light on the complex molecular interplay between cell surface GSLs, cholesterol and Abeta peptides, and on the influence of this molecular ballet on Abeta-membrane interactions.
Conflict of interest statement
Figures




References
-
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. - PubMed
-
- Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991;12:383–388. - PubMed
-
- Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. - PubMed
-
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

