Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non-immune pigs
- PMID: 20140096
- PMCID: PMC2816721
- DOI: 10.1371/journal.pone.0009068
Replication, pathogenesis and transmission of pandemic (H1N1) 2009 virus in non-immune pigs
Abstract
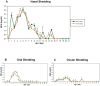
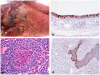
The declaration of the human influenza A pandemic (H1N1) 2009 (H1N1/09) raised important questions, including origin and host range [1], [2]. Two of the three pandemics in the last century resulted in the spread of virus to pigs (H1N1, 1918; H3N2, 1968) with subsequent independent establishment and evolution within swine worldwide [3]. A key public and veterinary health consideration in the context of the evolving pandemic is whether the H1N1/09 virus could become established in pig populations [4]. We performed an infection and transmission study in pigs with A/California/07/09. In combination, clinical, pathological, modified influenza A matrix gene real time RT-PCR and viral genomic analyses have shown that infection results in the induction of clinical signs, viral pathogenesis restricted to the respiratory tract, infection dynamics consistent with endemic strains of influenza A in pigs, virus transmissibility between pigs and virus-host adaptation events. Our results demonstrate that extant H1N1/09 is fully capable of becoming established in global pig populations. We also show the roles of viral receptor specificity in both transmission and tissue tropism. Remarkably, following direct inoculation of pigs with virus quasispecies differing by amino acid substitutions in the haemagglutinin receptor-binding site, only virus with aspartic acid at position 225 (225D) was detected in nasal secretions of contact infected pigs. In contrast, in lower respiratory tract samples from directly inoculated pigs, with clearly demonstrable pulmonary pathology, there was apparent selection of a virus variant with glycine (225G). These findings provide potential clues to the existence and biological significance of viral receptor-binding variants with 225D and 225G during the 1918 pandemic [5].
Conflict of interest statement
Figures
References
-
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
-
- Brown IH. Klenk H-D, Matrosovich MN, Stech J, editors. The role of pigs in interspecies transmission, in: ‘Avian Influenza’. Monogr Virol.; 2008. pp. 88–100. Basel Karger,
-
- Ma W, Lager KM, Vincent AL, Janke BH, Gramer MR, et al. The role of swine in the generation of novel influenza viruses. Zoonoses and Public Health 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

