TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii
- PMID: 20140195
- PMCID: PMC2816694
- DOI: 10.1371/journal.ppat.1000754
TgMORN1 is a key organizer for the basal complex of Toxoplasma gondii
Abstract
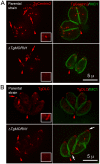
Toxoplasma gondii is a leading cause of congenital birth defects, as well as a cause for ocular and neurological diseases in humans. Its cytoskeleton is essential for parasite replication and invasion and contains many unique structures that are potential drug targets. Therefore, the biogenesis of the cytoskeletal structure of T. gondii is not only important for its pathogenesis, but also of interest to cell biology in general. Previously, we and others identified a new T. gondii cytoskeletal protein, TgMORN1, which is recruited to the basal complex at the very beginning of daughter formation. However, its function remained largely unknown. In this study, we generated a knock-out mutant of TgMORN1 (DeltaTgMORN1) using a Cre-LoxP based approach. We found that the structure of the basal complex was grossly affected in DeltaTgMORN1 parasites, which also displayed defects in cytokinesis. Moreover, DeltaTgMORN1 parasites showed significant growth impairment in vitro, and this translated into greatly attenuated virulence in mice. Therefore, our results demonstrate that TgMORN1 is required for maintaining the structural integrity of the parasite posterior end, and provide direct evidence that cytoskeleton integrity is essential for parasite virulence and pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
TgTKL1 Is a Unique Plant-Like Nuclear Kinase That Plays an Essential Role in Acute Toxoplasmosis.mBio. 2018 Mar 20;9(2):e00301-18. doi: 10.1128/mBio.00301-18. mBio. 2018. PMID: 29559568 Free PMC article.
-
Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii.PLoS Pathog. 2008 Jan;4(1):e10. doi: 10.1371/journal.ppat.0040010. PLoS Pathog. 2008. PMID: 18208326 Free PMC article.
-
Characterization of strain-specific phenotypes associated with knockout of dense granule protein 9 in Toxoplasma gondii.Mol Biochem Parasitol. 2019 Apr;229:53-61. doi: 10.1016/j.molbiopara.2019.01.003. Epub 2019 Mar 5. Mol Biochem Parasitol. 2019. PMID: 30849416
-
Genetic approaches for understanding virulence in Toxoplasma gondii.Brief Funct Genomics. 2011 Nov;10(6):365-73. doi: 10.1093/bfgp/elr028. Epub 2011 Sep 19. Brief Funct Genomics. 2011. PMID: 21930659 Review.
-
Roles of the tubulin-based cytoskeleton in the Toxoplasma gondii apical complex.Trends Parasitol. 2024 May;40(5):401-415. doi: 10.1016/j.pt.2024.02.010. Epub 2024 Mar 25. Trends Parasitol. 2024. PMID: 38531711 Review.
Cited by
-
Centrin2 from the human parasite Toxoplasma gondii is required for its invasion and intracellular replication.J Cell Sci. 2019 Jul 1;132(13):jcs228791. doi: 10.1242/jcs.228791. J Cell Sci. 2019. PMID: 31182647 Free PMC article.
-
Cell division in apicomplexan parasites.Nat Rev Microbiol. 2014 Feb;12(2):125-36. doi: 10.1038/nrmicro3184. Epub 2014 Jan 2. Nat Rev Microbiol. 2014. PMID: 24384598 Review.
-
Proteomic characterization of the Toxoplasma gondii cytokinesis machinery portrays an expanded hierarchy of its assembly and function.Nat Commun. 2022 Aug 8;13(1):4644. doi: 10.1038/s41467-022-32151-0. Nat Commun. 2022. PMID: 35941170 Free PMC article.
-
The Toxoplasma monocarboxylate transporters are involved in the metabolism within the apicoplast and are linked to parasite survival.Elife. 2024 Mar 19;12:RP88866. doi: 10.7554/eLife.88866. Elife. 2024. PMID: 38502570 Free PMC article.
-
Co-dependent formation of the Toxoplasma gondii sub-pellicular microtubules and inner membrane skeleton.bioRxiv [Preprint]. 2024 May 25:2024.05.25.595886. doi: 10.1101/2024.05.25.595886. bioRxiv. 2024. Update in: mBio. 2025 Aug 13:e0138925. doi: 10.1128/mbio.01389-25. PMID: 38826480 Free PMC article. Updated. Preprint.
References
-
- Levine ND. Progress in taxonomy of the Apicomplexan protozoa. J Protozool. 1988;35:518–520. - PubMed
-
- Zuckerman A. Recent studies on factors involved in malarial anemia. Military Medicine. 1966;131(Suppl):1201–1216. - PubMed
-
- Nagel RL. Malarial anemia. Hemoglobin. 2002;26:329–343. - PubMed
-
- Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, et al. Malaria, anemia, and malnutrition in african children–defining intervention priorities. Journal of Infectious Diseases. 2006;194:108–114. - PubMed
-
- Nichols BA, Chiappino ML. Cytoskeleton of Toxoplasma gondii. J Protozool. 1987;34:217–226. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

