Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice
- PMID: 20140197
- PMCID: PMC2816696
- DOI: 10.1371/journal.ppat.1000755
Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice
Abstract
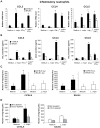
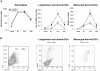
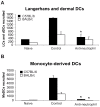
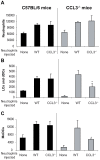
Neutrophils are rapidly and massively recruited to sites of microbial infection, where they can influence the recruitment of dendritic cells. Here, we have analyzed the role of neutrophil released chemokines in the early recruitment of dendritic cells (DCs) in an experimental model of Leishmania major infection. We show in vitro, as well as during infection, that the parasite induced the expression of CCL3 selectively in neutrophils from L. major resistant mice. Neutrophil-secreted CCL3 was critical in chemotaxis of immature DCs, an effect lost upon CCL3 neutralisation. Depletion of neutrophils prior to infection, as well as pharmacological or genetic inhibition of CCL3, resulted in a significant decrease in DC recruitment at the site of parasite inoculation. Decreased DC recruitment in CCL3(-/-) mice was corrected by the transfer of wild type neutrophils at the time of infection. The early release of CCL3 by neutrophils was further shown to have a transient impact on the development of a protective immune response. Altogether, we identified a novel role for neutrophil-secreted CCL3 in the first wave of DC recruitment to the site of infection with L. major, suggesting that the selective release of neutrophil-secreted chemokines may regulate the development of immune response to pathogens.
Conflict of interest statement
AEIP is an employee of Merck-Serono Research center in Geneva. All of the other authors declare no conflict of interest.
Figures



References
-
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Appelberg R. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 2007;15:87–92. - PubMed
-
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. - PubMed
-
- Beil WJ, Meinardus-Hager G, Neugebauer DC, Sorg C. Differences in the onset of the inflammatory response to cutaneous leishmaniasis in resistant and susceptible mice. J Leukoc Biol. 1992;52:135–142. - PubMed
-
- Tacchini-Cottier F, Zweifel C, Belkaid Y, Mukankundiye C, Vasei M, et al. An immunomodulatory function for neutrophils during the induction of a CD4+ Th2 response in BALB/c mice infected with Leishmania major. J Immunol. 2000;165:2628–2636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

