Exacerbated innate host response to SARS-CoV in aged non-human primates
- PMID: 20140198
- PMCID: PMC2816697
- DOI: 10.1371/journal.ppat.1000756
Exacerbated innate host response to SARS-CoV in aged non-human primates
Abstract
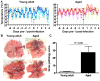
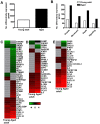
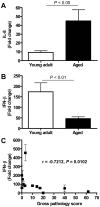
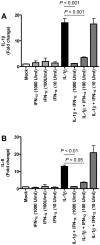
The emergence of viral respiratory pathogens with pandemic potential, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and influenza A H5N1, urges the need for deciphering their pathogenesis to develop new intervention strategies. SARS-CoV infection causes acute lung injury (ALI) that may develop into life-threatening acute respiratory distress syndrome (ARDS) with advanced age correlating positively with adverse disease outcome. The molecular pathways, however, that cause virus-induced ALI/ARDS in aged individuals are ill-defined. Here, we show that SARS-CoV-infected aged macaques develop more severe pathology than young adult animals, even though viral replication levels are similar. Comprehensive genomic analyses indicate that aged macaques have a stronger host response to virus infection than young adult macaques, with an increase in differential expression of genes associated with inflammation, with NF-kappaB as central player, whereas expression of type I interferon (IFN)-beta is reduced. Therapeutic treatment of SARS-CoV-infected aged macaques with type I IFN reduces pathology and diminishes pro-inflammatory gene expression, including interleukin-8 (IL-8) levels, without affecting virus replication in the lungs. Thus, ALI in SARS-CoV-infected aged macaques developed as a result of an exacerbated innate host response. The anti-inflammatory action of type I IFN reveals a potential intervention strategy for virus-induced ALI.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques.PLoS Pathog. 2007 Aug 10;3(8):e112. doi: 10.1371/journal.ppat.0030112. PLoS Pathog. 2007. PMID: 17696609 Free PMC article.
-
Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species.J Virol. 2011 May;85(9):4234-45. doi: 10.1128/JVI.02395-10. Epub 2011 Feb 16. J Virol. 2011. PMID: 21325418 Free PMC article.
-
Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection.JCI Insight. 2019 Feb 21;4(4):e123158. doi: 10.1172/jci.insight.123158. eCollection 2019 Feb 21. JCI Insight. 2019. PMID: 30830861 Free PMC article.
-
Inflammation, Thrombosis, and Destruction: The Three-Headed Cerberus of Trauma- and SARS-CoV-2-Induced ARDS.Front Immunol. 2020 Sep 25;11:584514. doi: 10.3389/fimmu.2020.584514. eCollection 2020. Front Immunol. 2020. PMID: 33101314 Free PMC article. Review.
-
Coronavirus virulence genes with main focus on SARS-CoV envelope gene.Virus Res. 2014 Dec 19;194:124-37. doi: 10.1016/j.virusres.2014.07.024. Epub 2014 Aug 2. Virus Res. 2014. PMID: 25093995 Free PMC article. Review.
Cited by
-
Drug repurposing of anti-infective clinical drugs: Discovery of two potential anti-cytokine storm agents.Biomed Pharmacother. 2020 Nov;131:110643. doi: 10.1016/j.biopha.2020.110643. Epub 2020 Aug 23. Biomed Pharmacother. 2020. PMID: 32846329 Free PMC article.
-
Virus discovery: one step beyond.Curr Opin Virol. 2013 Apr;3(2):e1-e6. doi: 10.1016/j.coviro.2013.03.007. Epub 2013 Apr 8. Curr Opin Virol. 2013. PMID: 23578938 Free PMC article.
-
Tackling the cytokine storm in COVID-19, challenges and hopes.Life Sci. 2020 Sep 15;257:118054. doi: 10.1016/j.lfs.2020.118054. Epub 2020 Jul 11. Life Sci. 2020. PMID: 32663575 Free PMC article. Review.
-
Of Mice and Men: The Coronavirus MHV and Mouse Models as a Translational Approach to Understand SARS-CoV-2.Viruses. 2020 Aug 12;12(8):880. doi: 10.3390/v12080880. Viruses. 2020. PMID: 32806708 Free PMC article. Review.
-
Can Adenosine Fight COVID-19 Acute Respiratory Distress Syndrome?J Clin Med. 2020 Sep 21;9(9):3045. doi: 10.3390/jcm9093045. J Clin Med. 2020. PMID: 32967358 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

