Transient dimers of allergens
- PMID: 20140203
- PMCID: PMC2816702
- DOI: 10.1371/journal.pone.0009037
Transient dimers of allergens
Abstract
Background: Allergen-mediated cross-linking of IgE antibodies bound to the FcepsilonRI receptors on the mast cell surface is the key feature of the type I allergy. If an allergen is a homodimer, its allergenicity is enhanced because it would only need one type of antibody, instead of two, for cross-linking.
Methodology/principal findings: An analysis of 55 crystal structures of allergens showed that 80% of them exist in symmetric dimers or oligomers in crystals. The majority are transient dimers that are formed at high protein concentrations that are reached in cells by colocalization. Native mass spectrometric analysis showed that native allergens do indeed form transient dimers in solution, while hypoallergenic variants of them exist almost solely in the monomeric form. We created a monomeric Bos d 5 allergen and show that it has a reduced capability to induce histamine release.
Conclusions/significance: The results suggest that dimerization would be a very common and essential feature for allergens. Thus, the preparation of purely monomeric variants of allergens could open up novel possibilities for specific immunotherapy.
Conflict of interest statement
Figures
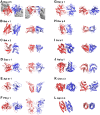
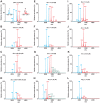
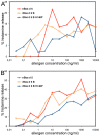
References
-
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature Rev Immunol. 2008;8:205–217. - PubMed
-
- Mari A, Scala E, Palazzo P, Ridolfi S, Zennaro D, et al. Bioinformatics applied to allergy: Allergen databases, from collecting sequence information to data integration. The allergome platform as a model. Cell Immunol. 2006;244:97–100. - PubMed
-
- Radauer C, Bublin M, Wagner S, Mari A, Breiteneder H. Allergens are distributed into few protein families and possess a restricted number of biochemical functions. J All Clin Immunol. 2008;121:847–852. - PubMed
-
- Niemi M, Jylhä S, Laukkanen ML, Söderlund H, Mäkinen-Kiljunen S, et al. Molecular interactions between a recombinant IgE antibody and the β-lactoglobulin allergen. Structure. 2007;15:1413–1421. - PubMed
-
- Nooren IMA, Thornton JM. Structural characterization and functional significance of transient protein-protein interactions. J Mol Biol. 2003;325:991–1018. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

