Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial
- PMID: 20140214
- PMCID: PMC2816207
- DOI: 10.1371/journal.pone.0009041
Safety and immunogenicity of an AMA1 malaria vaccine in Malian children: results of a phase 1 randomized controlled trial
Abstract
Background: The objective was to evaluate the safety and immunogenicity of the AMA1-based malaria vaccine FMP2.1/AS02(A) in children exposed to seasonal falciparum malaria.
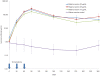
Methodology/principal findings: A Phase 1 double blind randomized controlled dose escalation trial was conducted in Bandiagara, Mali, West Africa, a rural town with intense seasonal transmission of Plasmodium falciparum malaria. The malaria vaccine FMP2.1/AS02(A) is a recombinant protein (FMP2.1) based on apical membrane antigen 1 (AMA1) from the 3D7 clone of P. falciparum, formulated in the Adjuvant System AS02(A). The comparator vaccine was a cell-culture rabies virus vaccine (RabAvert). One hundred healthy Malian children aged 1-6 years were recruited into 3 cohorts and randomized to receive either 10 microg FMP2.1 in 0.1 mL AS02(A), or 25 microg FMP2.1 in 0.25 mL AS02(A), or 50 microg FMP2.1 50 microg in 0.5 mL AS02(A), or rabies vaccine. Three doses of vaccine were given at 0, 1 and 2 months, and children were followed for 1 year. Solicited symptoms were assessed for 7 days and unsolicited symptoms for 30 days after each vaccination. Serious adverse events were assessed throughout the study. Transient local pain and swelling were common and more frequent in all malaria vaccine dosage groups than in the comparator group, but were acceptable to parents of participants. Levels of anti-AMA1 antibodies measured by ELISA increased significantly (at least 100-fold compared to baseline) in all 3 malaria vaccine groups, and remained high during the year of follow up.
Conclusion/significance: The FMP2.1/AS02(A) vaccine had a good safety profile, was well-tolerated, and induced high and sustained antibody levels in malaria-exposed children. This malaria vaccine is being evaluated in a Phase 2 efficacy trial in children at this site.
Trial registration: ClinicalTrials.gov NCT00358332 [NCT00358332].
Conflict of interest statement
Figures

Similar articles
-
Safety and immunogenicity of an AMA-1 malaria vaccine in Malian adults: results of a phase 1 randomized controlled trial.PLoS One. 2008 Jan 23;3(1):e1465. doi: 10.1371/journal.pone.0001465. PLoS One. 2008. PMID: 18213374 Free PMC article. Clinical Trial.
-
Phase 1 randomized controlled trial to evaluate the safety and immunogenicity of recombinant Pichia pastoris-expressed Plasmodium falciparum apical membrane antigen 1 (PfAMA1-FVO [25-545]) in healthy Malian adults in Bandiagara.Malar J. 2016 Aug 30;15(1):442. doi: 10.1186/s12936-016-1466-4. Malar J. 2016. PMID: 27577237 Free PMC article. Clinical Trial.
-
Extended safety, immunogenicity and efficacy of a blood-stage malaria vaccine in malian children: 24-month follow-up of a randomized, double-blinded phase 2 trial.PLoS One. 2013 Nov 18;8(11):e79323. doi: 10.1371/journal.pone.0079323. eCollection 2013. PLoS One. 2013. PMID: 24260195 Free PMC article. Clinical Trial.
-
Strain-specific Plasmodium falciparum multifunctional CD4(+) T cell cytokine expression in Malian children immunized with the FMP2.1/AS02A vaccine candidate.Vaccine. 2016 May 17;34(23):2546-55. doi: 10.1016/j.vaccine.2016.04.019. Epub 2016 Apr 15. Vaccine. 2016. PMID: 27087149 Clinical Trial.
-
Immunoglobulin G subclass and antibody avidity responses in Malian children immunized with Plasmodium falciparum apical membrane antigen 1 vaccine candidate FMP2.1/AS02A.Malar J. 2019 Jan 18;18(1):13. doi: 10.1186/s12936-019-2637-x. Malar J. 2019. PMID: 30658710 Free PMC article.
Cited by
-
Antibodies Against the Plasmodium vivax Apical Membrane Antigen 1 From the Belem Strain Share Common Epitopes Among Other Worldwide Variants.Front Cell Infect Microbiol. 2021 Mar 16;11:616230. doi: 10.3389/fcimb.2021.616230. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33796476 Free PMC article.
-
A Synthetic Nanoparticle Based Vaccine Approach Targeting MSP4/5 Is Immunogenic and Induces Moderate Protection Against Murine Blood-Stage Malaria.Front Immunol. 2019 Mar 15;10:331. doi: 10.3389/fimmu.2019.00331. eCollection 2019. Front Immunol. 2019. PMID: 30930890 Free PMC article.
-
Seroreactivity to Plasmodium falciparum erythrocyte membrane protein 1 intracellular domain in malaria-exposed children and adults.J Infect Dis. 2013 Nov 1;208(9):1514-9. doi: 10.1093/infdis/jit339. Epub 2013 Jul 30. J Infect Dis. 2013. PMID: 23901079 Free PMC article.
-
Lack of allele-specific efficacy of a bivalent AMA1 malaria vaccine.Malar J. 2010 Jun 21;9:175. doi: 10.1186/1475-2875-9-175. Malar J. 2010. PMID: 20565971 Free PMC article. Clinical Trial.
-
Structure-based design of a strain transcending AMA1-RON2L malaria vaccine.Nat Commun. 2023 Sep 2;14(1):5345. doi: 10.1038/s41467-023-40878-7. Nat Commun. 2023. PMID: 37660103 Free PMC article.
References
-
- Kocken CH, van der Wel AM, Dubbeld MA, Narum DL, van de Rijke FM, et al. Precise timing of expression of a Plasmodium falciparum-derived transgene in Plasmodium berghei is a critical determinant of subsequent subcellular localization. J Biol Chem. 1998;273:15119–15124. - PubMed
-
- Thomas AW, Deans JA, Mitchell GH, Alderson T, Cohen S. The Fab fragments of monoclonal IgG to a merozoite surface antigen inhibit Plasmodium knowlesi invasion of erythrocytes. Mol Biochem Parasitol. 1984;13:187–199. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

