Ribosylation rapidly induces alpha-synuclein to form highly cytotoxic molten globules of advanced glycation end products
- PMID: 20140223
- PMCID: PMC2816216
- DOI: 10.1371/journal.pone.0009052
Ribosylation rapidly induces alpha-synuclein to form highly cytotoxic molten globules of advanced glycation end products
Abstract
Background: Alpha synuclein (alpha-Syn) is the main component of Lewy bodies which are associated with several neurodegenerative diseases such as Parkinson's disease. While the glycation with D-glucose that results in alpha-Syn misfold and aggregation has been studied, the effects of glycation with D-ribose on alpha-Syn have not been investigated.
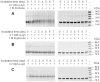
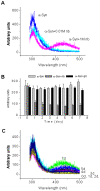
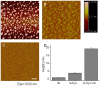
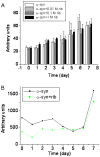
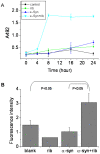
Methodology/principal findings: Here, we show that ribosylation induces alpha-Syn misfolding and generates advanced glycation end products (AGEs) which form protein molten globules with high cytotoxcity. Results from native- and SDS-PAGE showed that D-ribose reacted rapidly with alpha-Syn, leading to dimerization and polymerization. Trypsin digestion and sequencing analysis revealed that during ribosylation the lysinyl residues (K(58), K(60), K(80), K(96), K(97) and K(102)) in the C-terminal region reacted more quickly with D-ribose than those of the N-terminal region. Using Western blotting, AGEs resulting from the glycation of alpha-Syn were observed within 24 h in the presence of D-ribose, but were not observed in the presence of D-glucose. Changes in fluorescence at 410 nm demonstrated again that AGEs were formed during early ribosylation. Changes in the secondary structure of ribosylated alpha-Syn were not clearly detected by CD spectrometry in studies on protein conformation. However, intrinsic fluorescence at 310 nm decreased markedly in the presence of D-ribose. Observations with atomic force microscopy showed that the surface morphology of glycated alpha-Syn looked like globular aggregates. thioflavin T (ThT) fluorescence increased during alpha-Syn incubation regardless of ribosylation. As incubation time increased, ribosylation of alpha-Syn resulted in a blue-shift (approximately 100 nm) in the fluorescence of ANS. The light scattering intensity of ribosylated alpha-Syn was not markedly different from native alpha-Syn, suggesting that ribosylated alpha-Syn is present as molten protein globules. Ribosylated products had a high cytotoxicity to SH-SY5Y cells, leading to LDH release and increase in the levels of reactive oxygen species (ROS).
Conclusions/significance: alpha-Syn is rapidly glycated in the presence of D-ribose generating molten globule-like aggregations which cause cell oxidative stress and result in high cytotoxicity.
Conflict of interest statement
Figures







Similar articles
-
Rapid glycation with D-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells.BMC Cell Biol. 2009 Feb 13;10:10. doi: 10.1186/1471-2121-10-10. BMC Cell Biol. 2009. PMID: 19216769 Free PMC article.
-
Ribosylation of bovine serum albumin induces ROS accumulation and cell death in cancer line (MCF-7).Eur Biophys J. 2013 Dec;42(11-12):811-8. doi: 10.1007/s00249-013-0929-6. Epub 2013 Nov 12. Eur Biophys J. 2013. PMID: 24218080
-
D-Ribosylated Tau forms globular aggregates with high cytotoxicity.Cell Mol Life Sci. 2009 Aug;66(15):2559-71. doi: 10.1007/s00018-009-0058-7. Epub 2009 Jun 11. Cell Mol Life Sci. 2009. PMID: 19517062 Free PMC article.
-
Alpha-synuclein at the intracellular and the extracellular side: functional and dysfunctional implications.Biol Chem. 2017 Jan 1;398(1):77-100. doi: 10.1515/hsz-2016-0201. Biol Chem. 2017. PMID: 27508962 Review.
-
D-ribose in glycation and protein aggregation.Biochim Biophys Acta. 2012 Apr;1820(4):488-94. doi: 10.1016/j.bbagen.2012.01.005. Epub 2012 Jan 17. Biochim Biophys Acta. 2012. PMID: 22274132 Review.
Cited by
-
Unravelling the effect of N(ε)-(carboxyethyl)lysine on the conformation, dynamics and aggregation propensity of α-synuclein.Chem Sci. 2020 Mar 10;11(12):3332-3344. doi: 10.1039/d0sc00906g. Chem Sci. 2020. PMID: 34122841 Free PMC article.
-
DJ-1 Acts as a Scavenger of α-Synuclein Oligomers and Restores Monomeric Glycated α-Synuclein.Biomolecules. 2021 Oct 6;11(10):1466. doi: 10.3390/biom11101466. Biomolecules. 2021. PMID: 34680099 Free PMC article.
-
Alpha-Synuclein Glycation and the Action of Anti-Diabetic Agents in Parkinson's Disease.J Parkinsons Dis. 2018;8(1):33-43. doi: 10.3233/JPD-171285. J Parkinsons Dis. 2018. PMID: 29480231 Free PMC article. Review.
-
Proteome wide reduction in AGE modification in streptozotocin induced diabetic mice by hydralazine mediated transglycation.Sci Rep. 2013 Oct 15;3:2941. doi: 10.1038/srep02941. Sci Rep. 2013. PMID: 24126953 Free PMC article.
-
An overview on glycation: molecular mechanisms, impact on proteins, pathogenesis, and inhibition.Biophys Rev. 2024 Apr 12;16(2):189-218. doi: 10.1007/s12551-024-01188-4. eCollection 2024 Apr. Biophys Rev. 2024. PMID: 38737201 Free PMC article. Review.
References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, et al. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Barzilai A, Melamed E. Molecular mechanisms of selective dopaminergic neuronal death in Parkinson's disease. Trends Mol Med. 2003;9:126–132. - PubMed
-
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. - PubMed
-
- Iwatsubo T. Pathological biochemistry of alpha-synucleinopathy. Neuropathology. 2007;27:474–478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

