F-actin binding regions on the androgen receptor and huntingtin increase aggregation and alter aggregate characteristics
- PMID: 20140226
- PMCID: PMC2816219
- DOI: 10.1371/journal.pone.0009053
F-actin binding regions on the androgen receptor and huntingtin increase aggregation and alter aggregate characteristics
Abstract
Protein aggregation is associated with neurodegeneration. Polyglutamine expansion diseases such as spinobulbar muscular atrophy and Huntington disease feature proteins that are destabilized by an expanded polyglutamine tract in their N-termini. It has previously been reported that intracellular aggregation of these target proteins, the androgen receptor (AR) and huntingtin (Htt), is modulated by actin-regulatory pathways. Sequences that flank the polyglutamine tract of AR and Htt might influence protein aggregation and toxicity through protein-protein interactions, but this has not been studied in detail. Here we have evaluated an N-terminal 127 amino acid fragment of AR and Htt exon 1. The first 50 amino acids of ARN127 and the first 14 amino acids of Htt exon 1 mediate binding to filamentous actin in vitro. Deletion of these actin-binding regions renders the polyglutamine-expanded forms of ARN127 and Htt exon 1 less aggregation-prone, and increases the SDS-solubility of aggregates that do form. These regions thus appear to alter the aggregation frequency and type of polyglutamine-induced aggregation. These findings highlight the importance of flanking sequences in determining the propensity of unstable proteins to misfold.
Conflict of interest statement
Figures
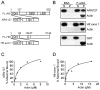
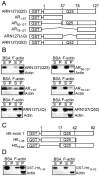
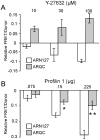

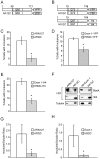
References
-
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–79. - PubMed
-
- A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971–983. - PubMed
-
- Li M, Chevalier-Larsen ES, Merry DE, Diamond MI. Soluble androgen receptor oligomers underlie pathology in a mouse model of spinobulbar muscular atrophy. J Biol Chem. 2007;282:3157–3164. - PubMed
-
- Goldberg YP, Nicholson DW, Rasper DM, Kalchman MA, Koide HB, et al. Cleavage of huntingtin by apopain, a proapoptotic cysteine protease, is modulated by the polyglutamine tract. Nat Genet. 1996;13:442–449. - PubMed
-
- Li M, Miwa S, Kobayashi Y, Merry DE, Yamamoto M, et al. Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol. 1998;44:249–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

