Dynamics of the bacterial intermediate filament crescentin in vitro and in vivo
- PMID: 20140233
- PMCID: PMC2816638
- DOI: 10.1371/journal.pone.0008855
Dynamics of the bacterial intermediate filament crescentin in vitro and in vivo
Abstract
Background: Crescentin, the recently discovered bacterial intermediate filament protein, organizes into an extended filamentous structure that spans the length of the bacterium Caulobacter crescentus and plays a critical role in defining its curvature. The mechanism by which crescentin mediates cell curvature and whether crescentin filamentous structures are dynamic and/or polar are not fully understood.
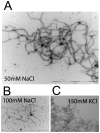
Methodology/principal findings: Using light microscopy, electron microscopy and quantitative rheology, we investigated the mechanics and dynamics of crescentin structures. Live-cell microscopy reveals that crescentin forms structures in vivo that undergo slow remodeling. The exchange of subunits between these structures and a pool of unassembled subunits is slow during the life cycle of the cell however; in vitro assembly and gelation of C. crescentus crescentin structures are rapid. Moreover, crescentin forms filamentous structures that are elastic, solid-like, and, like other intermediate filaments, can recover a significant portion of their network elasticity after shear. The assembly efficiency of crescentin is largely unaffected by monovalent cations (K(+), Na(+)), but is enhanced by divalent cations (Mg(2+), Ca(2+)), suggesting that the assembly kinetics and micromechanics of crescentin depend on the valence of the ions present in solution.
Conclusions/significance: These results indicate that crescentin forms filamentous structures that are elastic, labile, and stiff, and that their low dissociation rate from established structures controls the slow remodeling of crescentin in C. crescentus.
Conflict of interest statement
Figures



Similar articles
-
Filament structure and subcellular organization of the bacterial intermediate filament-like protein crescentin.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2309984121. doi: 10.1073/pnas.2309984121. Epub 2024 Feb 7. Proc Natl Acad Sci U S A. 2024. PMID: 38324567 Free PMC article.
-
The domain organization of the bacterial intermediate filament-like protein crescentin is important for assembly and function.Cytoskeleton (Hoboken). 2011 Apr;68(4):205-19. doi: 10.1002/cm.20505. Epub 2011 Mar 4. Cytoskeleton (Hoboken). 2011. PMID: 21360832 Free PMC article.
-
Bacterial cell curvature through mechanical control of cell growth.EMBO J. 2009 May 6;28(9):1208-19. doi: 10.1038/emboj.2009.61. Epub 2009 Mar 12. EMBO J. 2009. PMID: 19279668 Free PMC article.
-
Intermediate filament-like cytoskeleton of Caulobacter crescentus.J Mol Microbiol Biotechnol. 2006;11(3-5):152-8. doi: 10.1159/000094051. J Mol Microbiol Biotechnol. 2006. PMID: 16983192 Review.
-
Intermediate Filaments Supporting Cell Shape and Growth in Bacteria.Subcell Biochem. 2017;84:161-211. doi: 10.1007/978-3-319-53047-5_6. Subcell Biochem. 2017. PMID: 28500526 Review.
Cited by
-
Cytoskeletal Proteins in Caulobacter crescentus: Spatial Orchestrators of Cell Cycle Progression, Development, and Cell Shape.Subcell Biochem. 2017;84:103-137. doi: 10.1007/978-3-319-53047-5_4. Subcell Biochem. 2017. PMID: 28500524 Free PMC article. Review.
-
Filament structure and subcellular organization of the bacterial intermediate filament-like protein crescentin.Proc Natl Acad Sci U S A. 2024 Feb 13;121(7):e2309984121. doi: 10.1073/pnas.2309984121. Epub 2024 Feb 7. Proc Natl Acad Sci U S A. 2024. PMID: 38324567 Free PMC article.
-
Viscoelastic behavior of human lamin A proteins in the context of dilated cardiomyopathy.PLoS One. 2013 Dec 30;8(12):e83410. doi: 10.1371/journal.pone.0083410. eCollection 2013. PLoS One. 2013. PMID: 24386194 Free PMC article.
-
Growth of curved and helical bacterial cells.Soft Matter. 2012 Jul 28;8(28):7446-7451. doi: 10.1039/C2SM25452B. Soft Matter. 2012. PMID: 26120350 Free PMC article.
-
Intermediate filaments of the lung.Histochem Cell Biol. 2013 Jul;140(1):65-9. doi: 10.1007/s00418-013-1105-x. Epub 2013 Jun 14. Histochem Cell Biol. 2013. PMID: 23765163 Review.
References
-
- Ausmees N, Kuhn JR, Jacobs-Wagner C. The bacterial cytoskeleton: an intermediate filament-like function in cell shape. Cell. 2003;115:705–713. - PubMed
-
- Jones LJ, Carballido-Lopez R, Errington J. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell. 2001;104:913–922. - PubMed
-
- Moller-Jensen J, Borch J, Dam M, Jensen RB, Roepstorff P, et al. Bacterial mitosis: ParM of plasmid R1 moves plasmid DNA by an actin-like insertional polymerization mechanism. Mol Cell. 2003;12:1477–1487. - PubMed
-
- Soufo HJ, Graumann PL. Actin-like proteins MreB and Mbl from Bacillus subtilis are required for bipolar positioning of replication origins. Curr Biol. 2003;13:1916–1920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

