DNA binding of centromere protein C (CENPC) is stabilized by single-stranded RNA
- PMID: 20140237
- PMCID: PMC2816676
- DOI: 10.1371/journal.pgen.1000835
DNA binding of centromere protein C (CENPC) is stabilized by single-stranded RNA
Abstract
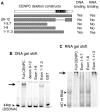
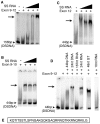
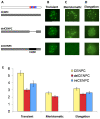
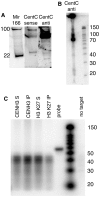
Centromeres are the attachment points between the genome and the cytoskeleton: centromeres bind to kinetochores, which in turn bind to spindles and move chromosomes. Paradoxically, the DNA sequence of centromeres has little or no role in perpetuating kinetochores. As such they are striking examples of genetic information being transmitted in a manner that is independent of DNA sequence (epigenetically). It has been found that RNA transcribed from centromeres remains bound within the kinetochore region, and this local population of RNA is thought to be part of the epigenetic marking system. Here we carried out a genetic and biochemical study of maize CENPC, a key inner kinetochore protein. We show that DNA binding is conferred by a localized region 122 amino acids long, and that the DNA-binding reaction is exquisitely sensitive to single-stranded RNA. Long, single-stranded nucleic acids strongly promote the binding of CENPC to DNA, and the types of RNAs that stabilize DNA binding match in size and character the RNAs present on kinetochores in vivo. Removal or replacement of the binding module with HIV integrase binding domain causes a partial delocalization of CENPC in vivo. The data suggest that centromeric RNA helps to recruit CENPC to the inner kinetochore by altering its DNA binding characteristics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Centromeric localization of αKNL2 and CENP-C proteins in plants depends on their centromere-targeting domain and DNA-binding regions.Nucleic Acids Res. 2025 Feb 8;53(4):gkae1242. doi: 10.1093/nar/gkae1242. Nucleic Acids Res. 2025. PMID: 39718987 Free PMC article.
-
A maize homolog of mammalian CENPC is a constitutive component of the inner kinetochore.Plant Cell. 1999 Jul;11(7):1227-38. doi: 10.1105/tpc.11.7.1227. Plant Cell. 1999. PMID: 10402425 Free PMC article.
-
Targeting of Arabidopsis KNL2 to Centromeres Depends on the Conserved CENPC-k Motif in Its C Terminus.Plant Cell. 2017 Jan;29(1):144-155. doi: 10.1105/tpc.16.00720. Epub 2017 Jan 6. Plant Cell. 2017. PMID: 28062749 Free PMC article.
-
DNA and proteins of plant centromeres.Curr Opin Plant Biol. 2003 Dec;6(6):554-60. doi: 10.1016/j.pbi.2003.09.007. Curr Opin Plant Biol. 2003. PMID: 14611953 Review.
-
Transcribing Centromeres: Noncoding RNAs and Kinetochore Assembly.Trends Genet. 2018 Aug;34(8):587-599. doi: 10.1016/j.tig.2018.05.001. Epub 2018 Jun 2. Trends Genet. 2018. PMID: 29871772 Review.
Cited by
-
The Genomic Landscape of Centromeres in Cancers.Sci Rep. 2019 Aug 2;9(1):11259. doi: 10.1038/s41598-019-47757-6. Sci Rep. 2019. PMID: 31375789 Free PMC article.
-
Epigenetic regulation of centromere function.Cell Mol Life Sci. 2020 Aug;77(15):2899-2917. doi: 10.1007/s00018-020-03460-8. Epub 2020 Feb 1. Cell Mol Life Sci. 2020. PMID: 32008088 Free PMC article. Review.
-
Repetitive centromeric satellite RNA is essential for kinetochore formation and cell division.J Cell Biol. 2014 Nov 10;207(3):335-49. doi: 10.1083/jcb.201404097. Epub 2014 Nov 3. J Cell Biol. 2014. PMID: 25365994 Free PMC article.
-
Genome-wide mapping reveals R-loops associated with centromeric repeats in maize.Genome Res. 2021 Aug;31(8):1409-1418. doi: 10.1101/gr.275270.121. Epub 2021 Jul 9. Genome Res. 2021. PMID: 34244230 Free PMC article.
-
Genetic drivers and cellular selection of female mosaic X chromosome loss.Nature. 2024 Jul;631(8019):134-141. doi: 10.1038/s41586-024-07533-7. Epub 2024 Jun 12. Nature. 2024. PMID: 38867047
References
-
- Yu HG, Hiatt EN, Dawe RK. The plant kinetochore. Trends Plant Sci. 2000;5:543–547. - PubMed
-
- Kotwaliwale C, Biggins S. Microtubule capture: a concerted effort. Cell. 2006;127:1105–1108. - PubMed
-
- Fukagawa T. The kinetochore and spindle checkpoint in vertebrate cells. Front Biosci. 2008;13:2705–2713. - PubMed
-
- Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293:1098–1102. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

