Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke
- PMID: 20140243
- PMCID: PMC2815773
- DOI: 10.1371/journal.pone.0008433
Genetically-defined deficiency of mannose-binding lectin is associated with protection after experimental stroke in mice and outcome in human stroke
Abstract
Background: The complement system is a major effector of innate immunity that has been involved in stroke brain damage. Complement activation occurs through the classical, alternative and lectin pathways. The latter is initiated by mannose-binding lectin (MBL) and MBL-associated serine proteases (MASPs). Here we investigated whether the lectin pathway contributes to stroke outcome in mice and humans.
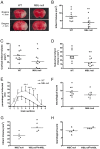
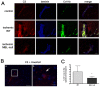
Methodology/principal findings: Focal cerebral ischemia/reperfusion in MBL-null mice induced smaller infarctions, better functional outcome, and diminished C3 deposition and neutrophil infiltration than in wild-type mice. Accordingly, reconstitution of MBL-null mice with recombinant human MBL (rhMBL) enhanced brain damage. In order to investigate the clinical relevance of these experimental observations, a study of MBL2 and MASP-2 gene polymorphism rendering the lectin pathway dysfunctional was performed in 135 stroke patients. In logistic regression adjusted for age, gender and initial stroke severity, unfavourable outcome at 3 months was associated with MBL-sufficient genotype (OR 10.85, p = 0.008) and circulating MBL levels (OR 1.29, p = 0.04). Individuals carrying MBL-low genotypes (17.8%) had lower C3, C4, and CRP levels, and the proinflammatory cytokine profile was attenuated versus MBL-sufficient genotypes.
Conclusions/significance: In conclusion, genetically defined MBL-deficiency is associated with a better outcome after acute stroke in mice and humans.
Conflict of interest statement
Figures




References
-
- Carroll MC, Holers VM. Innate autoimmunity. Adv Immunol. 2005;86:37–57. - PubMed
-
- van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci. 2003;992:56–71. - PubMed
-
- Mocco J, Wilson DA, Komotar RJ, Sughrue ME, Coates K, et al. Alterations in plasma complement levels after human ischemic stroke. Neurosurgery. 2006;59:28–33. - PubMed
-
- Lindsberg PJ, Ohman J, Lehto T, Karjalainen-Lindsberg ML, Paetau A, et al. Complement activation in the central nervous system following blood-brain barrier damage in man. Ann Neurol. 1996;40:587–596. - PubMed
-
- Frank MM, Fries LF. The role of complement in inflammation and phagocytosis. Immunol Today. 1991;12:322–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

