Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse
- PMID: 20140248
- PMCID: PMC2815778
- DOI: 10.1371/journal.pone.0009027
Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse
Abstract
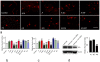
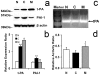
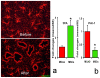
We demonstrate that tissue plasminogen activator (tPA) and its inhibitors contribute to neurite outgrowth in the central nervous system (CNS) after treatment of stroke with multipotent mesenchymal stromal cells (MSCs). In vivo, administration of MSCs to mice subjected to middle cerebral artery occlusion (MCAo) significantly increased activation of tPA and downregulated PAI-1 levels in the ischemic boundary zone (IBZ) compared with control PBS treated mice, concurrently with increases of myelinated axons and synaptophysin. In vitro, MSCs significantly increased tPA levels and concomitantly reduced plasminogen activator inhibitor 1 (PAI-1) expression in astrocytes under normal and oxygen and glucose deprivation (OGD) conditions. ELISA analysis of conditioned medium revealed that MSCs stimulated astrocytes to secrete tPA. When primary cortical neurons were cultured in the conditioned medium from MSC co-cultured astrocytes, these neurons exhibited a significant increase in neurite outgrowth compared to conditioned medium from astrocytes alone. Blockage of tPA with a neutralizing antibody or knock-down of tPA with siRNA significantly attenuated the effect of the conditioned medium on neurite outgrowth. Addition of recombinant human tPA into cortical neuronal cultures also substantially enhanced neurite outgrowth. Collectively, these in vivo and in vitro data suggest that the MSC mediated increased activation of tPA in astrocytes promotes neurite outgrowth after stroke.
Conflict of interest statement
Figures



Similar articles
-
Mesenchymal Stromal Cells Promote Axonal Outgrowth Alone and Synergistically with Astrocytes via tPA.PLoS One. 2016 Dec 13;11(12):e0168345. doi: 10.1371/journal.pone.0168345. eCollection 2016. PLoS One. 2016. PMID: 27959956 Free PMC article.
-
Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study).J Cereb Blood Flow Metab. 2011 Nov;31(11):2181-8. doi: 10.1038/jcbfm.2011.116. Epub 2011 Aug 10. J Cereb Blood Flow Metab. 2011. PMID: 21829213 Free PMC article.
-
Valproic acid induces astrocyte-dependent neurite outgrowth from cultured rat primary cortical neuron via modulation of tPA/PAI-1 activity.Glia. 2013 May;61(5):694-709. doi: 10.1002/glia.22463. Epub 2013 Feb 4. Glia. 2013. PMID: 23378038
-
The role of endogenous tissue-type plasminogen activator in neuronal survival after ischemic stroke: friend or foe?Cell Mol Life Sci. 2019 Apr;76(8):1489-1506. doi: 10.1007/s00018-019-03005-8. Epub 2019 Jan 17. Cell Mol Life Sci. 2019. PMID: 30656378 Free PMC article. Review.
-
The role of astrocytes in mediating exogenous cell-based restorative therapy for stroke.Glia. 2014 Jan;62(1):1-16. doi: 10.1002/glia.22585. Epub 2013 Nov 4. Glia. 2014. PMID: 24272702 Free PMC article. Review.
Cited by
-
Cell-based therapy for ischemic stroke.Expert Opin Biol Ther. 2013 Sep;13(9):1229-40. doi: 10.1517/14712598.2013.804507. Epub 2013 Jun 6. Expert Opin Biol Ther. 2013. PMID: 23738646 Free PMC article. Review.
-
Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth.Stem Cells. 2012 Jul;30(7):1556-64. doi: 10.1002/stem.1129. Stem Cells. 2012. PMID: 22605481 Free PMC article.
-
Treatment of TBI with collagen scaffolds and human marrow stromal cells increases the expression of tissue plasminogen activator.J Neurotrauma. 2011 Jul;28(7):1199-207. doi: 10.1089/neu.2010.1694. Epub 2011 May 16. J Neurotrauma. 2011. PMID: 21355820 Free PMC article.
-
Neuronal production of lipocalin-2 as a help-me signal for glial activation.Stroke. 2014 Jul;45(7):2085-92. doi: 10.1161/STROKEAHA.114.005733. Epub 2014 Jun 10. Stroke. 2014. PMID: 24916903 Free PMC article. Clinical Trial.
-
Mesenchymal Stromal Cells Promote Axonal Outgrowth Alone and Synergistically with Astrocytes via tPA.PLoS One. 2016 Dec 13;11(12):e0168345. doi: 10.1371/journal.pone.0168345. eCollection 2016. PLoS One. 2016. PMID: 27959956 Free PMC article.
References
-
- Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. - PubMed
-
- Li Y, Chen J, Zhang CL, Wang L, Lu D, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. - PubMed
-
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, et al. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002;22:275–279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

