High-precision radiosurgical dose delivery by interlaced microbeam arrays of high-flux low-energy synchrotron X-rays
- PMID: 20140254
- PMCID: PMC2815784
- DOI: 10.1371/journal.pone.0009028
High-precision radiosurgical dose delivery by interlaced microbeam arrays of high-flux low-energy synchrotron X-rays
Abstract
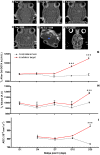
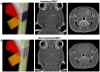
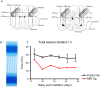
Microbeam Radiation Therapy (MRT) is a preclinical form of radiosurgery dedicated to brain tumor treatment. It uses micrometer-wide synchrotron-generated X-ray beams on the basis of spatial beam fractionation. Due to the radioresistance of normal brain vasculature to MRT, a continuous blood supply can be maintained which would in part explain the surprising tolerance of normal tissues to very high radiation doses (hundreds of Gy). Based on this well described normal tissue sparing effect of microplanar beams, we developed a new irradiation geometry which allows the delivery of a high uniform dose deposition at a given brain target whereas surrounding normal tissues are irradiated by well tolerated parallel microbeams only. Normal rat brains were exposed to 4 focally interlaced arrays of 10 microplanar beams (52 microm wide, spaced 200 microm on-center, 50 to 350 keV in energy range), targeted from 4 different ports, with a peak entrance dose of 200Gy each, to deliver an homogenous dose to a target volume of 7 mm(3) in the caudate nucleus. Magnetic resonance imaging follow-up of rats showed a highly localized increase in blood vessel permeability, starting 1 week after irradiation. Contrast agent diffusion was confined to the target volume and was still observed 1 month after irradiation, along with histopathological changes, including damaged blood vessels. No changes in vessel permeability were detected in the normal brain tissue surrounding the target. The interlacing radiation-induced reduction of spontaneous seizures of epileptic rats illustrated the potential pre-clinical applications of this new irradiation geometry. Finally, Monte Carlo simulations performed on a human-sized head phantom suggested that synchrotron photons can be used for human radiosurgical applications. Our data show that interlaced microbeam irradiation allows a high homogeneous dose deposition in a brain target and leads to a confined tissue necrosis while sparing surrounding tissues. The use of synchrotron-generated X-rays enables delivery of high doses for destruction of small focal regions in human brains, with sharper dose fall-offs than those described in any other conventional radiation therapy.
Conflict of interest statement
Figures




References
-
- Sims E, Doughty D, Macaulay E, Royle N, Wraith C, et al. Stereotactically delivered cranial radiation therapy: a ten-year experience of linac-based radiosurgery in the UK. Clin Oncol (R Coll Radiol) 1999;11:303–320. - PubMed
-
- St George EJ, Kudhail J, Perks J, Plowman PN. Acute symptoms after gamma knife radiosurgery. J Neurosurg. 2002;97:631–634. - PubMed
-
- Leksell L. The stereotactic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316–319. - PubMed
-
- Slatkin DN, Spanne P, Dilmanian FA, Sandborg M. Microbeam radiation therapy. Med Phys. 1992;19:1395–1400. - PubMed
-
- Slatkin DN, Dilmanian FA, Spanne P. Method for microbeam radiation therapy. United States 1994 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

