Melanoma cells treated with GGTI and IFN-gamma allow murine vaccination and enhance cytotoxic response against human melanoma cells
- PMID: 20140259
- PMCID: PMC2815789
- DOI: 10.1371/journal.pone.0009043
Melanoma cells treated with GGTI and IFN-gamma allow murine vaccination and enhance cytotoxic response against human melanoma cells
Abstract
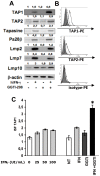
Background: Suboptimal activation of T lymphocytes by melanoma cells is often due to the defective expression of class I major histocompatibility antigens (MHC-I) and costimulatory molecules. We have previously shown that geranylgeranyl transferase inhibition (done with GGTI-298) stimulates anti-melanoma immune response through MHC-I and costimulatory molecule expression in the B16F10 murine model [1].
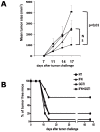
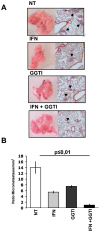
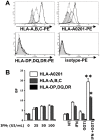
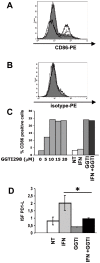
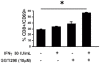
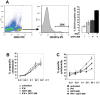
Methodology/principal findings: In this study, it is shown that vaccination with mIFN-gand GGTI-298 pretreated B16F10 cells induces a protection against untreated tumor growth and pulmonary metastases implantation. Furthermore, using a human melanoma model (LB1319-MEL), we demonstrated that in vitro treatment with hIFN-gamma and GGTI-298 led to the up regulation of MHC-I and a costimulatory molecule CD86 and down regulation of an inhibitory molecule PD-1L. Co-culture experiments with peripheral blood mononuclear cells (PBMC) revealed that modifications induced by hIFN-gamma and GGTI-298 on the selected melanoma cells, enables the stimulation of lymphocytes from HLA compatible healthy donors. Indeed, as compared with untreated melanoma cells, pretreatment with hIFN-gamma and GGTI-298 together rendered the melanoma cells more efficient at inducing the: i) activation of CD8 T lymphocytes (CD8+/CD69+); ii) proliferation of tumor-specific CD8 T cells (MelanA-MART1/TCR+); iii) secretion of hIFN-gamma; and iv) anti-melanoma specific cytotoxic cells.
Conclusions/significance: These data indicate that pharmacological treatment of melanoma cell lines with IFN-gamma and GGTI-298 stimulates their immunogenicity and could be a novel approach to produce tumor cells suitable for vaccination and for stimulation of anti-melanoma effector cells.
Conflict of interest statement
Figures

Similar articles
-
Geranylgeranyl transferase inhibition stimulates anti-melanoma immune response through MHC Class I and costimulatory molecule expression.FASEB J. 2005 Sep;19(11):1513-5. doi: 10.1096/fj.04-3482fje. Epub 2005 Jun 29. FASEB J. 2005. PMID: 15990392
-
CD80-mediated induction of immunostimulation in two ocular melanoma cell lines is augmented by interferon-gamma.Melanoma Res. 2002 Apr;12(2):129-38. doi: 10.1097/00008390-200204000-00005. Melanoma Res. 2002. PMID: 11930109
-
Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma.J Immunol. 2003 Dec 1;171(11):5940-7. doi: 10.4049/jimmunol.171.11.5940. J Immunol. 2003. PMID: 14634105
-
The duration of TCR/pMHC interactions regulates CTL effector function and tumor-killing capacity.Eur J Immunol. 2009 Aug;39(8):2259-69. doi: 10.1002/eji.200939341. Eur J Immunol. 2009. PMID: 19637198
-
Topical treatment of all-trans retinoic acid inhibits murine melanoma partly by promoting CD8+ T-cell immunity.Immunology. 2017 Oct;152(2):287-297. doi: 10.1111/imm.12768. Epub 2017 Jun 29. Immunology. 2017. PMID: 28556970 Free PMC article.
Cited by
-
Statin use and its effect on all-cause mortality of melanoma patients: a population-based Dutch cohort study.Cancer Med. 2014 Oct;3(5):1284-93. doi: 10.1002/cam4.285. Epub 2014 Jun 17. Cancer Med. 2014. PMID: 24935402 Free PMC article.
-
Melanoma Expressed-CD70 Is Regulated by RhoA and MAPK Pathways without Affecting Vemurafenib Treatment Activity.PLoS One. 2016 Feb 1;11(2):e0148095. doi: 10.1371/journal.pone.0148095. eCollection 2016. PLoS One. 2016. PMID: 26828592 Free PMC article.
-
Statins Reduce Melanoma Development and Metastasis through MICA Overexpression.Front Immunol. 2013 Mar 13;4:62. doi: 10.3389/fimmu.2013.00062. eCollection 2013. Front Immunol. 2013. PMID: 23493799 Free PMC article.
-
The cholesterol pathway: impact on immunity and cancer.Trends Immunol. 2022 Jan;43(1):78-92. doi: 10.1016/j.it.2021.11.007. Trends Immunol. 2022. PMID: 34942082 Free PMC article. Review.
-
KALRN mutations promote antitumor immunity and immunotherapy response in cancer.J Immunother Cancer. 2020 Oct;8(2):e000293. doi: 10.1136/jitc-2019-000293. J Immunother Cancer. 2020. PMID: 33037113 Free PMC article.
References
-
- Tilkin-Mariame AF, Cormary C, Ferro N, Sarrabayrouse G, Lajoie-Mazenc I, et al. Geranylgeranyl transferase inhibition stimulates anti-melanoma immune response through MHC Class I and costimulatory molecule expression. FASEB J. 2005;19:1513–1515. - PubMed
-
- Luo W, Ko E, Hsu JC, Wang X, Ferrone S. Targeting melanoma cells with human high molecular weight-melanoma associated antigen-specific antibodies elicited by a peptide mimotope: functional effects. J Immunol. 2006;176:6046–6054. - PubMed
-
- Akiyama Y, Maruyama K, Nara N, Mochizuki T, Yamamoto A, et al. Cytotoxic T cell induction against human malignant melanoma cells using HLA-A24-restricted melanoma peptide cocktail. Anticancer Res. 2004;24:571–577. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

