HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase
- PMID: 20140431
- PMCID: PMC3038205
- DOI: 10.1007/s00262-010-0820-6
HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase
Abstract
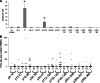
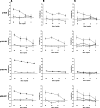
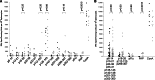
Prostatic acid phosphatase (PAP) has been investigated as the target of several antigen-specific anti-prostate tumor vaccines. The goal of antigen-specific active immunotherapies targeting PAP would ideally be to elicit PAP-specific CD8+ effector T cells. The identification of PAP-specific CD8+ T-cell epitopes should provide a means of monitoring the immunological efficacy of vaccines targeting PAP, and these epitopes might themselves be developed as vaccine antigens. In the current report, we hypothesized that PAP-specific epitopes might be identified by direct identification of pre-existing CD8+ T cells specific for HLA-A2-restricted peptides derived from PAP in the blood of HLA-A2-expressing individuals. 11 nonamer peptides derived from the amino acid sequence of PAP were used as stimulator antigens in functional ELISPOT assays with peripheral blood mononuclear cells from 20 HLA-A2+ patients with prostate cancer or ten healthy blood donors. Peptide-specific T cells were frequently identified in both groups for three of the peptides, p18-26, p112-120, and p135-143. CD8+ T-cell clones specific for three peptides, p18-26, p112-120, and p299-307, confirmed that these are HLA-A2-restricted T-cell epitopes. Moreover, HLA-A2 transgenic mice immunized with a DNA vaccine encoding PAP developed epitope-specific responses for one or more of these three peptide epitopes. We propose that this method to first identify epitopes for which there are pre-existing epitope-specific T cells could be used to prioritize MHC class I-specific epitopes for other antigens. In addition, we propose that the epitopes identified here could be used to monitor immune responses in HLA-A2+ patients receiving vaccines targeting PAP to identify potentially therapeutic immune responses.
Figures
References
-
- Siddall JK, Cooper EH, Newling DWW, Robinson MRG, Whelan P. An evaluation of the immunochemical measurement of prostatic acid phosphatase and prostatic specific antigen in carcinoma of the prostate. Eur Urol. 1986;12:123–130. - PubMed
-
- Laus R, Yang DM, Ruegg CL, Shapero MH, Slagle PH, Small E, Burch P, Valone FH. Dendritic cell immunotherapy of prostate cancer: preclinical models and early clinical experience. Canc Res Ther Control. 2001;11:1–10.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

