Maitake beta-glucan promotes recovery of leukocytes and myeloid cell function in peripheral blood from paclitaxel hematotoxicity
- PMID: 20140432
- PMCID: PMC3268513
- DOI: 10.1007/s00262-009-0815-3
Maitake beta-glucan promotes recovery of leukocytes and myeloid cell function in peripheral blood from paclitaxel hematotoxicity
Abstract
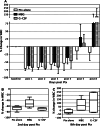
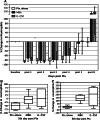
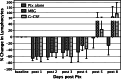
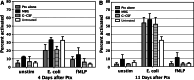
Bone marrow myelotoxicity is a major limitation of chemotherapy. While granulocyte colony stimulating factor (G-CSF) treatment is effective, alternative approaches to support hematopoietic recovery are sought. We previously found that a beta-glucan extract from maitake mushroom Grifola frondosa (MBG) enhanced colony forming unit-granulocyte monocyte (CFU-GM) activity of mouse bone marrow and human hematopoietic progenitor cells (HPC), stimulated G-CSF production and spared HPC from doxorubicin toxicity in vitro. This investigation assessed the effects of MBG on leukocyte recovery and granulocyte/monocyte function in vivo after dose intensive paclitaxel (Ptx) in a normal mouse. After a cumulative dose of Ptx (90-120 mg/kg) given to B6D2F1mice, daily oral MBG (4 or 6 mg/kg), intravenous G-CSF (80 microg/kg) or Ptx alone were compared for effects on the dynamics of leukocyte recovery in blood, CFU-GM activity in bone marrow and spleen, and granulocyte/monocyte production of reactive oxygen species (ROS). Leukocyte counts declined less in Ptx + MBG mice compared to Ptx-alone (p = 0.024) or Ptx + G-CSF treatment (p = 0.031). Lymphocyte levels were higher after Ptx + MBG but not Ptx + G-CSF treatment compared to Ptx alone (p < 0.01). MBG increased CFU-GM activity in bone marrow and spleen (p < 0.001, p = 0.002) 2 days after Ptx. After two additional days (Ptx post-day 4), MBG restored granulocyte/monocyte ROS response to normal levels compared to Ptx-alone and increased ROS response compared to Ptx-alone or Ptx + G-CSF (p < 0.01, both). The studies indicate that oral MBG promoted maturation of HPC to become functionally active myeloid cells and enhanced peripheral blood leukocyte recovery after chemotoxic bone marrow injury.
Figures


References
-
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, Davidson NE, Martino S, Livingston R, Ingle JN, Perez EA, Carpenter J, Hurd D, Holland JF, Smith BL, Sartor CI, Leung EH, Abrams J, Schilsky RL, Muss HB, Norton L. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. - DOI - PubMed
-
- De Boer RH, Eisen TG, Ellis PA, Johnston SR, Walsh G, Ashley S, Smith IE. A randomised phase II study of conventional versus accelerated infusional chemotherapy with granulocyte colony-stimulating factor support in advanced breast cancer. Ann Oncol. 2002;13:889–894. doi: 10.1093/annonc/mdf150. - DOI - PubMed
-
- Osma MM, Ortuno F, Lozano ML, Gomez-Espuch J, Ayala F, Sanchez-Serrano I, Perez-Ceballos E, Moraleda JM, Vicente V. Administration of post-autologous PBSCT rhG-CSF is associated with long-term low concentrations of bone marrow hematopoietic progenitor cells. Bone Marrow Transplant. 2001;27:1287–1292. doi: 10.1038/sj.bmt.1703079. - DOI - PubMed
-
- Pape H, Orth K, Heese A, Heyll A, Kobbe G, Schmitt G, Niederbichler AD, Peiper M, Schwarz A, Boelke E. G-CSF during large field radiotherapy reduces bone marrow recovery capacity. Eur J Med Res. 2006;11:322–328. - PubMed
