Immobilization of 293 cells using porous support particles for adenovirus vector production
- PMID: 20140496
- PMCID: PMC2978301
- DOI: 10.1007/s10616-010-9254-4
Immobilization of 293 cells using porous support particles for adenovirus vector production
Abstract
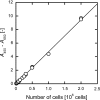
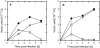
Adenovirus vector production by anchorage-independent 293 cells immobilized using porous biomass support particles (BSPs) was investigated in static and shake-flask cultures for efficient large-scale production of adenovirus vectors for gene therapy applications. The density of cells immobilized within BSPs was evaluated by measuring their WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt) reduction activity. In shake-flask culture, 293-F cells, which were adapted to serum-free suspension culture, were not successfully retained within reticulated polyvinyl formal (PVF) resin BSPs (2 × 2 × 2 mm cubes) with matrices of relatively small pores (pore diameter 60 μm). When the BSPs were coated with a cationic polymer polyethyleneimine, a high cell density of more than 10(7) cells cm(-3)-BSP was achieved in both static and shake-flask cultures with regular replacement of the culture medium. After infection with an adenovirus vector carrying the enhanced green fluorescent protein gene (Ad EGFP), the specific Ad EGFP productivity of the immobilized cells was comparable to the maximal productivity of non-immobilized 293-F cells by maintaining favorable conditions in the culture environment.
Figures

Similar articles
-
Production of recombinant protein by baculovirus-infected insect cells in immobilized culture using porous biomass support particles.J Biosci Bioeng. 2000;89(1):12-7. doi: 10.1016/s1389-1723(00)88044-5. J Biosci Bioeng. 2000. PMID: 16232692
-
Immobilization of Escherichia coli cells using porous support particles coated with cationic polymers.J Biosci Bioeng. 2007 Aug;104(2):98-103. doi: 10.1263/jbb.104.98. J Biosci Bioeng. 2007. PMID: 17884653
-
Growth and death behaviour of anchorage-independent animal cells immobilized within porous support matrices.Appl Microbiol Biotechnol. 1992 May;37(2):244-51. doi: 10.1007/BF00178179. Appl Microbiol Biotechnol. 1992. PMID: 1368243
-
A fibrous-bed bioreactor for continuous production of monoclonal antibody by hybridoma.Adv Biochem Eng Biotechnol. 2004;87:61-96. doi: 10.1007/b94364. Adv Biochem Eng Biotechnol. 2004. PMID: 15217104 Review.
-
Practices of shake-flask culture and advances in monitoring CO2 and O2.Appl Microbiol Biotechnol. 2018 May;102(10):4279-4289. doi: 10.1007/s00253-018-8922-8. Epub 2018 Mar 26. Appl Microbiol Biotechnol. 2018. PMID: 29582104 Review.
References
-
- Gene Therapy Clinical Trials Worldwide (2009) The Journal of Gene Medicine. Wiley, Hoboken. http://www.wiley.co.uk/genmed/clinical/. Cited 1 July 2009
LinkOut - more resources
Full Text Sources

