Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter
- PMID: 20140636
- PMCID: PMC5169165
- DOI: 10.1007/s11095-009-9983-2
Regulation of human organic anion transporter 4 by protein kinase C and NHERF-1: altering the endocytosis of the transporter
Abstract
Purpose: Human organic anion transporter 4 (hOAT4) belongs to a family of organic anion transporters that play critical roles in the body disposition of clinically important drugs. We have previously shown that the activity of hOAT4 was down-regulated by activation of PKC and up-regulated by PDZ protein NHERF-1. Here, we investigated the mechanisms underlying such regulations.
Methods: COS-7 cells expressing hOAT4 were treated with PKC activator phorbol 12-myristate 13-acetate (PMA) or transfected with dominant negative mutants of dynamin-2 or Eps15 or transfected with NHERF-1. The internalization and the function of hOAT4 were then determined.
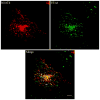
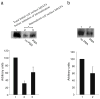
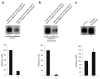
Results: We showed that hOAT4 constitutively internalized from and recycled back to plasma membrane. Transfection of dominant negative mutants of dynamin-2 or Eps15 into the cells, all of which block clathrin-dependent endocytotic pathway, significantly blocked hOAT4 internalization. Treatment of cells with PMA accelerated hOAT4 internalization, whereas transfection of cells with NHERF-1 attenuated hOAT4 internalization.
Conclusion: Our studies demonstrated that i) hOAT4 undergoes constitutive trafficking between cell surface and intracellular compartments, ii) hOAT4 internalization partly occurs through clathrin-dependent pathway, iii) the down-regulation of hOAT4 activity by activation of PKC and the up-regulation of hOAT4 activity by NHERF-1 are mediated through alteration of hOAT4 internalization.
Figures
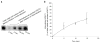

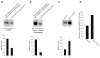
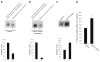
Similar articles
-
Organic anion transporter OAT1 undergoes constitutive and protein kinase C-regulated trafficking through a dynamin- and clathrin-dependent pathway.J Biol Chem. 2008 Nov 21;283(47):32570-9. doi: 10.1074/jbc.M800298200. Epub 2008 Sep 25. J Biol Chem. 2008. PMID: 18818201 Free PMC article.
-
Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells.Eur J Pharm Sci. 2006 Apr;27(5):518-23. doi: 10.1016/j.ejps.2005.09.008. Epub 2005 Oct 27. Eur J Pharm Sci. 2006. PMID: 16257192
-
Regulation of human organic anion transporter 4 by progesterone and protein kinase C in human placental BeWo cells.Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E57-61. doi: 10.1152/ajpendo.00696.2006. Epub 2007 Mar 6. Am J Physiol Endocrinol Metab. 2007. PMID: 17341544
-
Signal complex regulation of renal transport proteins: NHERF and regulation of NHE3 by PKA.Am J Physiol Renal Physiol. 2000 Sep;279(3):F393-9. doi: 10.1152/ajprenal.2000.279.3.F393. Am J Physiol Renal Physiol. 2000. PMID: 10966919 Review.
-
Drug Transporters and Na+/H+ Exchange Regulatory Factor PSD-95/Drosophila Discs Large/ZO-1 Proteins.Pharmacol Rev. 2015 Jul;67(3):656-80. doi: 10.1124/pr.115.010728. Pharmacol Rev. 2015. PMID: 26092975 Free PMC article. Review.
Cited by
-
Regulation of human organic anion transporter 4 by parathyroid hormone-related protein and protein kinase A.Int J Biochem Mol Biol. 2012;3(3):322-7. Epub 2012 Sep 25. Int J Biochem Mol Biol. 2012. PMID: 23097748 Free PMC article.
-
Uraemic syndrome of chronic kidney disease: altered remote sensing and signalling.Nat Rev Nephrol. 2019 May;15(5):301-316. doi: 10.1038/s41581-019-0111-1. Nat Rev Nephrol. 2019. PMID: 30728454 Free PMC article. Review.
-
Posttranslational Regulation of Organic Anion Transporters by Ubiquitination: Known and Novel.Med Res Rev. 2016 Sep;36(5):964-79. doi: 10.1002/med.21397. Epub 2016 Jun 12. Med Res Rev. 2016. PMID: 27291023 Free PMC article. Review.
-
Is It Time to Use Modeling of Cellular Transporter Homeostasis to Inform Drug-Drug Interaction Studies: Theoretical Considerations.AAPS J. 2021 Aug 25;23(5):102. doi: 10.1208/s12248-021-00635-4. AAPS J. 2021. PMID: 34435271 Free PMC article.
-
The mechanistic links between insulin and human organic anion transporter 4.Int J Pharm. 2019 Jan 30;555:165-174. doi: 10.1016/j.ijpharm.2018.11.040. Epub 2018 Nov 16. Int J Pharm. 2019. PMID: 30453017 Free PMC article.
References
-
- Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: biopharmaceutical, physiological, and pathological roles. Pharm Res. 2007;24:450–70. - PubMed
-
- Zhou F, You G. Molecular insights into the structure-function relationship of organic anion transporters OATs. Pharm Res. 2007;24:28–36. - PubMed
-
- Anzai N, Kanai Y, Endou H. Organic anion transporter family: current knowledge. J Pharmacol Sci. 2006;100:411–26. - PubMed
-
- Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, et al. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94:297–304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

