Selective inhibition of human leukemia cell growth and induction of cell cycle arrest and apoptosis by pseudolaric acid B
- PMID: 20140742
- PMCID: PMC11827977
- DOI: 10.1007/s00432-010-0784-0
Selective inhibition of human leukemia cell growth and induction of cell cycle arrest and apoptosis by pseudolaric acid B
Abstract
Purpose: The leukemias account for the largest number of cases of childhood cancer and remain the primary cause of cancer-related mortality among children in the United States. There is a need for novel antileukemia agents due to toxicity and resistant to existing chemotherapeutic agents. In this study, the effects of pseudolaric acid B (PAB) on three human leukemia cell lines, acute promyelocytic leukemia HL-60 cells, acute lymphoblastic leukemia CCRF-CEM cells, and human chronic myeloid leukemia blast-phase K562 cells were investigated in vitro, compared to normal human peripheral blood mononuclear cells (PBMC).
Methods: Cell viability was determined using CellTiter-Glo luminescent reagent. Colony formation was assessed by Microtitration cloning assay. Cell cycle analysis was carried out by flow cytometry. Tubulin polymerization was measured by recording the increase in absorbance. Inhibition of topoisomerase I (topo I) and topoisomerase II (topo II) enzyme activities was measured by DNA relaxation assay using topo I and II drug screening kit. Apoptosis was observed by DAPI staining assay and Caspase3/7 activities was measured using Caspase-Glo((R)) 3/7 assay kit.
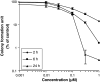
Results: Pseudolaric acid B selectively inhibited the growth of human leukemia HL-60, CCRF-CEM and K562 cells, but not normal PBMC. PAB suppressed colony formation in HL-60 cells. Cell cycle analysis showed that PAB blocked the cell cycle at G(2)/M phase in HL-60 cells, suggesting that it suppresses mitosis. DNA topo I and topo II were not inhibited, but tubulin polymerization was inhibited. PAB-induced apoptosis and activated caspase-3/7 activity.
Conclusions: This study indicates that PAB has a potential for use against leukemia and its effects might be mediated by inhibiting tubulin polymerization, preventing cell division and activating caspase-3, which leads to apoptosis.
Figures






References
-
- Cragg G, Suffness M (1988) Metabolism of plant-derived anticancer agents. Pharmacol Ther 37:425–461. doi:10.1016/0163-7258(88)90006-X - PubMed
-
- Cragg GM, Newman DJ, Snader KM (1997) Natural products in drug discovery and development. J Nat Prod 60:52–60. doi:10.1021/np9604893 - PubMed
-
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10:369–377. doi:10.1016/S0962-8924(00)01803-1 - PubMed
-
- Gerber DE (2008) Targeted therapies: a new generation of cancer treatments. Am Fam Physician 77:311–319 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

