Estrogen augments the T cell-dependent but not the T-independent immune response
- PMID: 20140748
- PMCID: PMC11115714
- DOI: 10.1007/s00018-010-0270-5
Estrogen augments the T cell-dependent but not the T-independent immune response
Erratum in
- Cell Mol Life Sci. 2010 Jul;67(14):2509. Kövesdi, Dorottya [added]
Abstract
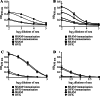
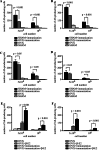
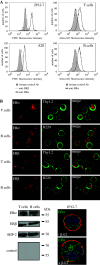
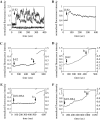
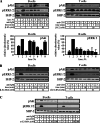
Estrogen plays a critical regulatory role in the development and maintenance of immunity. Its role in the regulation of antibody synthesis in vivo is still not completely clear. Here, we have compared the effect of estrogen on T cell-dependent (TD) and T cell-independent type 2 (TI-2) antibody responses. The results provide the first evidence that estrogen enhances the TD but not the TI-2 response. Ovariectomy significantly decreased, while estrogen re-administration increased the number of hapten-specific IgM- and IgG-producing cells in response to TD antigen. In vitro experiments also show that estrogen may have a direct impact on B and T cells by inducing rapid signaling events, such as Erk and AKT phosphorylation, cell-specific Ca(2+) signal, and NFkappaB activation. These non-transcriptional effects are mediated by classical estrogen receptors and partly by an as yet unidentified plasma membrane estrogen receptor. Such receptor- mediated rapid signals may modulate the in vivo T cell-dependent immune response.
Figures


References
-
- Grimaldi CM, Hicks R, Diamond B. B cell selection and susceptibility to autoimmunity. J Immunol. 2005;174:1775–1781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

